Avoiding pressure transmitter failure
Pressure measurement is often a critical element in process environments. For consistency and reliability, for the durability of equipment, and for the safety of everyone involved, pressure must be considered. In batch processes, pressure transmitters perform the vital function of tracking the pressure of these closed systems.
However, during cleaning cycles between batches, these transmitters can become compromised from the extreme heat of the steam-in-place cycle. Exposure to the high pressure and temperatures during thermocycling can cause errors in the transmitter data. This transmitter drift may throw off the measurement enough to produce inconsistent batches and put the process at risk of falling out of compliance with US Food and Drug Administration standards.
Historically, when pressure transmitters fail, they must be recalibrated to ensure performance is back to within specification or they need to be replaced. Either scenario costs money and valuable time. Advanced hygienic pressure measurement instrumentation is now able to withstand many clean-in-place (CIP) and sterilization-in-place (SIP) cycles, dramatically reducing transmitter drift and providing long-term stability to pressure measurement performance.
Pressure: A key indicator in the biopharma batch process
Batch processes are used in a wide variety of life sciences and food and beverage applications. Drug manufacturing and bioprocessing users depend on different types of batch processes for these biomanufacturing operations that produce pharmaceutical products like vaccines, commodity chemicals, and even specialized cellular tissue. Bioreactors are commonly used, often made of stainless steel, to support the required biologically active environment. They provide cells with a homogeneous environment and keep temperature, pH, and oxygen levels consistent. In this closed system, particular conditions must be established and maintained to preserve quality from batch to batch. The environment must remain sterile, and conditions have to be tightly controlled and monitored throughout the entire process.
Hygienic pressure transmitters are used in a range of reactor types, like fed-batch or continuous-stir tank bioreactors to continuously monitor the pressure of these systems. In the absence of proper pressure measurement, a number of significant issues could arise. Tanks can overflow or overpressure can rise too high, causing safety concerns and loss of product. Chemical reactions may occur at suboptimal conditions, not only causing the bioprocess to underperform but potentially leading to lower product quality or total batch loss. Inadequate offgas filtration can lead to wasted product or force the need for additional processing.
Batch bioprocesses are completed with only the cells and the sterile nutrient medium provided at the start of cultivation. For enzyme production, enzymes and their substrates are introduced at the start of the process. No other nutrients are added during the entire process making contamination from other substances extremely unlikely. Once the process is complete, the biomass or medium is harvested and the reactor must be cleaned and sterilized to undergo the process again.
There are many advantages to utilizing batch processes, but they depend on a highly controlled and sterile environment to yield results. Because each batch is its own individual production, it’s extremely important for manufacturers to be able to count on the viability of every single batch. They need repeatable and accurate results to ensure consistent product quality and production efficiency.
The challenges of thermocycling for hygienic transmitters
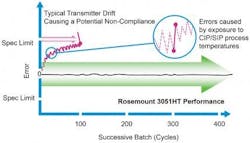
After a batch process is complete, the CIP and SIP cycles are run to fully clear the system of any biological materials from the previous batch, returning the reactor back to a sterilized state ready to begin a new batch. These cycles involve running high temperature fluids through the process equipment for cleaning and sterilization. These temperatures can reach as high as 300 degrees F and affect sensing devices throughout the reactor in different ways. When the pressure transmitter is subjected to these more extreme temperatures, it can become offset and errors can be introduced into the measurement data (see Figure 1).
Figure 1
Advanced hygienic pressure transmitters are able to withstand many CIP/SIP cycles with minimal drifting to minimize variability between batches.
The transmitter requires recovery time to get back to its normal performance range. When the pressure transmitter comes back online after a steam-in-place cycle, it begins to stabilize. However, it may not initially return to working pressure specifications and only gradually return to meeting specifications over an extended period of time. It may never actually return to the original calibrated settings without performing costly maintenance to recalibrate the device.
For legacy technology, there is no way to know how quickly the measurement data from a pressure transmitter can be trusted once it has recovered from the cleaning and sterilization process. How many times can the CIP and SIP cycles be run before the transmitter is out of spec and no longer viable as a measurement point? Can this critical pressure data be trusted? Does the industry standard include constant recalibration and replacement of this equipment after every cycle?
Safety, reliability and batch-to-batch repeatability
When thermocycling interferes with the transmitter’s ability to compensate for temperature while transmitting accurate pressure readings during the normal batch process, the safety and reliability of the entire process is compromised. If instrumentation stops functioning, production risks non-compliance with strict FDA rules and regulations. In cases like this, the batch could be marked for non-conformance and thrown out, or the SIP cycle may need to be redone to ensure that production has the right data to move forward with a new batch once again.
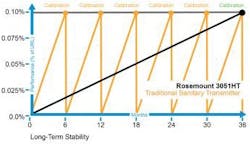
What an efficient operation needs is hygienic pressure transmitters that can withstand the cleaning cycles necessary to maintain a sanitary environment. To maximize batch production, these transmitters need to recover quickly after a steam-in-place or washdown cycle takes place. Ideally, pressure transmitters would perform these functions without production managers being forced to constantly recalibrate or replace the devices. Fewer recalibrations or in-line replacements of devices lessens the probability of batch delays (see Figure 2).
Robust batch-to-batch repeatability creates less process downtime between batches, increased batch consistency, and fewer maintenance calibrations. This drives significant potential for cost savings and improvement in batch quality.
Figure 2
When compared to traditional hygienic instrumentation, advanced pressure transmitters can reduce downtime and operating costs due to long-term stability that requires fewer calibrations.
Highly-engineered solutions for pharma
Fortunately, the technology to provide this cutting-edge functionality in hygienic pressure transmitters has been designed and tested in the field. Updated hygienic transmitter platforms are uniquely engineered with the challenges of sanitary applications in mind. Built-in advanced sensors enable robust batch-to-batch repeatability of up to ±0.02 percent of the upper range limit for 60 batches with less recovery time and longer maintenance intervals.
The sophisticated capabilities of the modern hygienic transmitter platform include easy-to-install sanitary fittings and specifications that meet 3-A Sanitary Standards as well as European Hygienic Engineering and Design Group (EHEDG) and American Society of Mechanical Engineers: Bioprocessing Equipment (ASME BPE) industry standards. The platform can tolerate washdown cycles up to 302 F (150 C) with minimal drifting and recovers 20-30 times faster than legacy equipment. Advanced diagnostics help prevent on-scale failures, and an intuitive local operator interface simplifies installation and commissioning.
The key to these advancements lies in the proven technology of pressure modules tested in the harshest conditions across multiple industries. This state-of-the-art technology has been modified to withstand wet environments and extreme washdown conditions without compromising the performance of the transmitter. Having been specifically engineered to tolerate CIP and SIP cycles without compromising accuracy and reliability, these innovative, modern hygienic pressure instruments are at the forefront of pressure technology today.