The pharma industry is heavily based on consumer and community trust and therefore has always been a highly regulated business where there are strict requirements for safety and quality. However, pharma is now subject to new business pressures, which include the need for more effective asset management.
Customer expectations are increasing, regulatory compliance is becoming stricter, patents expire, biosimilars emerge, alternative treatments are developed, and more powerful, competitive collaborative business models evolve. Discovering, developing, and supplying safe, effective medicine is being performed at a faster pace now.
Technology is also advancing at a more rapid pace. Many business functions are influenced by this global digital transformation — also called Pharma 4.0 — which has special implications for this highly regulated industry. Much emphasis is now placed on using data and analytics to drive efficiency and quality in production. The ultimate effect of this new business pressure is to ensure even more safety and quality in production. This is where effective asset management becomes even more important. Machine condition monitoring, a vital part of asset management, is now playing a much greater role in pharma than ever before.
Asset management in the digital transformation
As part of the digital transformation and Pharma 4.0 sweeping across the industry, new data solutions portfolios for collecting, enriching, storing, and accessing reliable, real-time operations data are bringing many benefits to the industry and play an important role for the asset management side of the business.
This enables condition monitoring to be performed from an enterprise level, thus minimizing the need for proprietary condition-monitoring servers. In addition to monitoring alarm limits, trending and notification of events, analytics can be performed on the data, making machine health care monitoring more transparent, reliable and efficient.
How monitoring pharma machines differs
Although there’s a difference in the processes monitored from one pharma manufacturer to the next, there are asset management issues that are similar across the entire pharma industry, yet quite unique when compared to other industries. In a pharma plant, there are a number of machines that are critical in manufacturing medicinal drugs, but unlike in other industries, the machines in the utilities and HVAC section of the pharma plant are just as critical as the production machines.
Utility systems and machines monitored
The utilities are the foundation of all pharma processes and represent the lifeblood of the plant. Production processes are completely dependent on these utilities.
If the water pumps, air compressors or power-generating units are not working as they should, production will simply stop. Therefore, these utility assets must be effectively monitored all the time, not just at intervals. Moreover, these assets are not the only critical machines to be monitored in the plant.
Heating, ventilation and air conditioning
The HVAC is one of the most important functions in the pharma plant. The primary purpose is to control the ambient environment around processes. It is also a function where 50-80% of all the energy is used.
The HVAC components must perform consistently and reliably to avoid the possibility of contamination and temperature and humidity deviations that could result in quarantined inventory or lost batches. Even a minor deviation of flow or a temperature moving out of specification can ruin a batch or result in additional processing. Such losses can be extremely expensive, however, many of these losses and problems are avoidable with effective condition monitoring and control. The table below summarizes the systems that are being monitored.
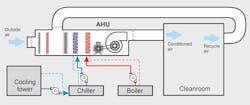
The most important component of the HVAC system, which is the very heart of the system, is the air handling unit (AHU). It provides a constant stream of filtered air to the cleanroom manufacturing area, controlling the humidity, temperature, air pressure and the amount of contaminants without any fluctuation. Product quality is highly dependent on it. Even though it is not part of the manufacturing process, if the AHU goes down, many of the processes served by the AHU will be interrupted because these processes are so dependent on precise environmental conditions.
Figure 2. AHU blower and motor
If the AHU blower fails, all processes served by the cleanroom have to be stopped. Therefore, monitoring this system is absolutely crucial in the pharma plant, but there are challenges that go with it. Because airflow has to be carefully regulated in the AHU, variable speed drive motors (VSD) have to be used. This is similar to many of the other processes in the plant that also use VSD motors. This poses a challenge for early fault detection and diagnostics for conventional monitoring systems and requires special monitoring techniques.
Production
Pharmaceutical production is basically milling (reducing particle size), granulation (combining particles), hot melt extrusion (active ingredient dispersion for easier delivery), coating, and tablet pressing. Many of these processes require strict temperature, humidity, air purity and material flow control, which can be jeopardized by a machine fault.
Many processes in a pharma plant are complicated enough to perform in a laboratory setting but become even more complex for industrial production. Precise temperature control, as previously mentioned, is very important but it can be difficult to achieve on an industrial scale. Some processes involve stoichiometric amounts of reagents to be added for the proper reaction, where if the machinery is not properly operating or controlled, the mixing can result in failed batches. Constant material flow rate is crucial for proper blending of the APIs and excipients.
The table below lists some of the machines being monitored in a production section of a plant. A typical plant has 60 pump/compressor machine trains and generating units that are monitored in the utilities section, 50 pump, compressor and fan machine trains in the HVAC section, and 72 production machine trains.
Asset management pays off
Asset management and reliability have recently become very important to the pharma industry to improve production and still maintain high standards for safety and product quality. Certain condition monitoring solutions can fit well into requirements for data management, early fault detection and reliable diagnostics.
Pharma customers often decide to monitor all the critical machines in the plant based on their need to reap maximum benefits for the entire plant for reduced downtime, less maintenance cost, and fewer lost batches. For so many machines being monitored, the cumulative maintenance cost savings is significant. More importantly, avoiding lost batches due to machine faults results in more savings. In many cases, the system can pay off after only preventing a couple of batch losses.