In modern process management, the lifecycle focuses on the total costs of the process from investment to operation to retirement. The pharmaceutical industry has begun to apply this concept to the lifecycle of an analytical method — consisting of design, development, validation (including instrumental qualification, continuous method performance verification, and method transfer), and retirement of the method.
Method lifecycle management (MLCM) provides an opportunity to use the knowledge gained from the application of scientific and quality risk management to provide continuous improvement and assurance of data quality. Regulatory bodies have also increased their focus on lifecycle management for analytical methods. Improvement of analytical methods based on performance is quickly becoming a compliance expectation.
Regulatory landscape
Both the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), as well as the United States Pharmacopeia (USP) Forum are developing new guidelines that include lifecycle management of analytical methods. The implementation of the ICH guidelines Q8 to Q11 within the pharmaceutical industry is intended to modernize the current approach for the development and manufacturing of pharmaceuticals.
The proposed ICH guideline, Q12, will provide guidance to facilitate the management of post-approval changes in a more predictable and efficient manner across the product lifecycle. It has been drafted to create a more harmonized approach to technical and regulatory considerations for method lifecycle management, as ICH Q8, Q9, Q10 and Q11 take more science and risk-based approaches to assessing change across lifecycles.
Possible impacts of Q12 include:
- Harmonization of change management
- Facilitatation of risk-based regulatory oversight and optimization of resources for review and inspection
- Assisting industry in maintaining dossier conformance while facilitating continual product and process improvement
- Enhanced use of regulatory tools for prospective change management
Adoption of this new ICH guideline will promote innovation and continual improvement, and strengthen quality assurance and the reliable supply of a product. In addition, other new ICH guidelines and a new general chapter of the USP are in the works to provide specific guidance about the method lifecycle management approach. Together, the new pieces of guidance will provide a more strategic approach to MLCM across the product lifecycle.
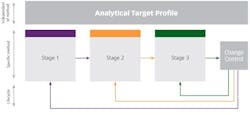
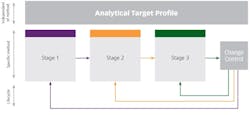
The three main stages of MLCM are as follows:
- Stage 1: Procedure design and development
- Stage 2: Procedure performance qualification
- Stage 3: Continued procedure performance verification
The role of an Analytical Target Profile
Setting an Analytical Target Profile (ATP) is a crucial part of the MLCM approach. A predefined, written record of the requirements of an analytical method, it lays down the performance requirement for the analytical procedure. It must be linked to the purpose of the method rather than to an analytical technique, and should be based on the intended use of the analytical method.
An ATP defines what is being measured and provides performance level characteristics. Having established the baseline, the ATP can then be used to push forward with the design and development of analytical methods.
According to a USP validation and verification expert panel, the following attributes should be considered when establishing an ATP:
- The sample being tested
- The matrix in which the analyte will be present
- An allowance for error of the measurement, assessed via accuracy and precision
- An allowance for risk of the criteria not being met
- An assurance that the measurement uncertainty and risk criteria are met
The ATP can therefore be used not only to check how suitable the analytical procedure is in the early stages of drug development, but also throughout the experiment. ICH Q10 outlines a requirement for “the application of a holistic quality management system,” which is where an established ATP holds great value. It is a point of reference that can be used throughout the lifecycle if the need for change arises, and applying this approach provides assurance that the quality objectives are being met throughout.
Applying Quality by Design principles
Once the ATP has been defined, the method development and evaluation process can begin. Method lifecycle management combines activities of analytical method development, improvement, qualification, validation, transfer, and maintenance related to Good Manufacturing Practice (GMP) production. Under this umbrella is the analytical Quality by Design (AQbD) approach, which can be used as an early risk assessment tool to identify method parameters that have an impact on analytical performance, as well as risks associated with variability, such as sample preparation, instrument configuration and environmental conditions. Helping to improve the robustness of manufacturing processes, AQbD also facilitates continuous development strategies for enhanced product quality and productivity in manufacturing, which is why pharmaceutical manufacturers and regulatory agencies have a shared interest in applying these principles to products and processes.
The result is more than a method — it is a quality tool. Using AQbD yields robust methods that will meet future requirements and developments, indicating not only the design space that an application will work in, but also where it will not.
With methods developed via AQbD, the impact of possible variables over a method’s lifetime has already been considered, so the need for revalidation is minimized. The result is a systematic approach that includes defining the method’s goal, assessing risk assessment, developing a design space, implementing a control strategy, and continually working on improvements to increase method robustness and knowledge.
Rather than looking at one factor at a time, which typically involves the optimization of one factor while the others remain constant, AQbD introduces multivariate analysis. This approach allows an overall understanding of method performance based on the multidimensional combination and interaction of these factors to be obtained, and it leads to the definition of the optimum design space. A greater understanding of the method variables leads to more robust and reliable methods, thereby increasing their fitness for purpose throughout their lifecycle.
Established methods are often ineffective, slow or lack robustness, which leads to an accumulation of out of specification results. Using AQbD, it is easy to show that approximately half of failures occur because a method is not robust, and the approach allows you to achieve significant gains in this area with a very small change. The challenge is finding out what the problem is and knowing what to change.
The pharmaceutical industry has learned to use regulations like a checklist. The vision of the AQbD concept was too complicated at first but, in time, the industry has been able to see how clear the documentation in an AQbD product is, which means it gets approved faster. The novelty and opportunity in this approach for the pharmaceutical industry is that working within the design space of a specific method is seen as an adjustment, and not a post-approval change. With the regulatory authorities often overwhelmed with small changes to documentation, implementing AQbD would significantly reduce the frequency of requests for these minor amends. In turn, regulators would be able to focus on more significant changes or more important topics, benefitting the pharmaceutical industry, authorities and the consumer.
Creating a compliant lab
Increasing understanding of methods developed with AQbD means that pharmaceutical service providers using the approach have been able to show that the number of failures and transfer issues that occur over the lifecycle of the method are often reduced. Typically, clients can achieve a 50 percent cost saving compared with standard quality control methods because AQbD results in robust analysis techniques with minimal associated quality control costs and significantly shortened analysis run times. For commercial processes, the high quality of the data provided by AQbD methods may also allow for more timely data release, reduced regulatory risk and lower costs.
Innovative instrumentation and software is particularly advantageous in troubleshooting methods because such tools need to be highly robust, dependable, and reproducible, even for the most demanding separations. In the laboratory, the troubleshooting process begins by performing a robustness test which often results in developing a new method. The development strategy follows AQbD principles and can be divided into five steps:
- Definition of method goals
- Risk assessment
- Design of experiments with screening and optimization steps
- Design space that includes model building, working point selection, and verification as method validation
- Method control strategy based on the knowledge gained about the developed method
The prospect of changing an already approved analytical method can make pharmaceutical companies nervous, but it is easy to show how the issue can be solved by taking an AQbD approach. There is also greater regulatory flexibility because results that fall within the well-defined design space are not considered to be changes in the method.
There is a role for pharmaceutical companies and instrument manufacturers in the proactive education of customers about the benefits of MLCM and AQbD — particularly how these approaches can be used under the current guidelines to develop methods that are robust, user-oriented, designed for routine analysis, easily transferable to any laboratory, and time- and cost-efficient.
The adoption of technology that can provide more information faster and earlier in the method development process is fundamental, as is software that can handle the data and ensure its integrity, which is critical to MLCM. Data trending is the backbone of an AQbD approach, by enabling timely identification and actions, controlling variability and risk, and serving as the basis for continuous improvement during the product lifecycle.
Using instruments and software that enable laboratories to understand how sources of variability impact method performance is very important, and this data trending contributes to reducing risk, lowering costs, and improving regulatory compliance.
Advancing Modern Medicine
MLCM and AQbD are set to change the way the pharmaceutical industry brings products to market, as global regulatory bodies continue to develop new guidelines that specifically address lifecycle management of analytical methods. The paradigm shift of MLCM moves the focus of pharmaceutical method development from regulatory compliance back to the scientific advances that are the catalysts for advancing the field of modern medicine.