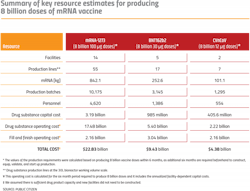
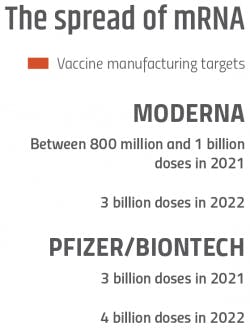
Early in the pandemic, the prospects of an mRNA candidate winning the vaccine race seemed dim. In fact, in April 2020, an AI program developed by the analytical firm Clarivate assigned Moderna’s mRNA vaccine a measly 5% chance of success.
But as the world would soon find out, the naysayers had it wrong about this nascent technology. Despite the fact that other mRNA products had lingered in clinical trials for years and none had yet to be approved for human use, this new approach to delivering immunity to a rapidly spreading pandemic had, in fact, found its moment to shine.
Now, with the potential of mRNA finally realized, companies throughout the industry — from drug developers to manufacturers — are quickly mobilizing to get in on the action.
“There is an avalanche coming of mRNA assets,” says Daniel Chancellor, Thought Leadership Director at Informa Pharma Intelligence. “This is an explosive area of the industry for investment and partnering.”
A glance at the current pipeline of mRNA products reveals this stunning growth. As of July, Informa Pharma Intelligence has identified 49 mRNA assets in clinical trials and 219 that have been reported in preclinical development (although there may be more because, as Chancellor puts it, many companies doing preclinical work prefer to “hold their cards close to their chest” and not reveal their research).
Over the course of just two months this year, 30 new assets were reported in the preclinical phases and overall, Chancellor says the pipeline has tripled in the space of two years.
Naturally, manufacturers, many of whom had only dabbled in the mRNA waters prior to the pandemic, are now readying themselves for the coming onslaught of new products.
“Pretty much every CMO is scaling up and investing in mRNA,” Chancellor says. “It’s a strategic imperative that they’ll need to be able to address for this kind of technology… and they’re all signaling partnerships and proclaiming expertise in that area.”
The sudden growth in mRNA during the pandemic — driven by the two authorized mRNA vaccines from Moderna and Pfizer/BioNTech — has also rippled through pharma’s supply chain from raw materials all the way down to the cold chain storage units needed to freeze the vaccines at ultracold temperatures.
“We were getting about 30-40 inquiries a month about our ULT25 freezer,” explains Scott Masiella, the director of Product Management at Stirling Ultracold, one of the industry’s primary suppliers of ultra-low temperature freezers. “During the pandemic, that increased to about 500-600 per month.”
Clearly, mRNA is the new big shot in the industry, and with the pressure on, vaccine developers were able to deliver. Yet, the scale-up of mRNA during the pandemic happened on the fly, and there’s no getting around the fact that the industry grappled with new challenges.
As pharma now braces for a wave of other mRNA products, manufacturers and distributors are still working through the kinks of large-scale production to improve the process before the mRNA market fully takes off.
The cool factor
In many ways, mRNA — messenger ribonucleic acid — is a dream platform for combatting a rapidly spreading virus.
Because mRNA vaccines use synthesized RNA to instruct the body’s cells to produce antigens, it is a non-infectious technology that eliminates the chances of spreading infection. The process of in vitro transcription (IVT) of DNA templates into mRNA strands also helps manufacturers achieve larger yields of higher potency vaccines — shrinking the manufacturing footprint.
“The low dosage takes strain off of a manufacturing process that is already easier to set up since the actual process is sequence-independent, and mRNA is relatively easy to produce as compared with recombinant protein, and live, attenuated virus vaccines,” Dr. Fouad Atouf, vice president of Global Biologics at U.S. Pharmacopeia, and others wrote1 in a published review of the vaccine platforms used to combat COVID-19.
As Atouf explained in a recent interview, the high yields are not just impressive in the landscape of vaccine platforms.
“The small amount per dose is amazing when you compare it to what you need to make for a monoclonal antibody (mAb) product — 10s of ug [micrograms] for mRNA vs. 100-1,000 ug for mAbs,” he says.
The mRNA sequence can also be changed to address new strains and viruses, making this type of vaccine easier to modify than conventional options. And once a production process is set up, the same manufacturing and purification procedures can be used for other types of mRNA vaccines — making it the “preferred platform for rapid response during a pandemic,” Atouf and others wrote.1
“The low footprint, relatively lower capital investment, agility and overall productivity of the platform has given the industry the capacity to quickly scale it up,” Amelie Boulais, head of Market Entry Strategy, Viral-based Therapeutics at Sartorius, says. “And we saw during COVID how fast that went.”
Drug developers are also embracing the limitless potential of mRNA beyond vaccines. Because cells use mRNA to translate the genes of DNA into proteins, therapies could potentially be created to express any protein and target any disease. Moderna’s president, Stephen Hoge, has, in fact, called mRNA the “software of life.”
“The mRNA platform is actually something that will become a big deal beyond vaccines. It will address many other indications in the future, such as cancer, cystic fibrosis and other conditions,” Atouf says.
But a big question remains as more mRNA products approach liftoff: How well will all of this potential play out in the manufacturing industry?
A bumpy ascent
The IVT reaction and purification
Because the scale-up of mRNA manufacturing is so recent, the industry has yet to adopt a standard platform for the process. But broadly speaking, making mRNA bulk drug substance for vaccines has followed this pattern:
- DNA plasmids containing the coronavirus spike protein gene can be multiplied by fermentation in a bacteria such as E. coli.
- The DNA plasmid is purified out with chromatography and filtration.
- Then in a reactor vessel, the plasmids are mixed with IVT reagents and enzymes to generate the mRNA, which is then purified and conditioned by filtrations and chromatography.
- The mRNA is capped and polyadenylated to improve stability and potency, and then purified, concentrated and conditioned some more.
- Finally, the mRNA is assembled into a delivery system, such as a lipid nanoparticle (LNP).
Before the pandemic struck, Boulais says that Sartorius, an industry partner for life science research and the biopharma industry, had already positioned itself in the mRNA market with a toolbox of manufacturing solutions for various mRNA production steps including mRNA capture, polishing, concentration and diafiltration, and storage.
As the industry has scaled up, Boulais says the company is seeing opportunities for improvement in multiple parts of the process. For example, when it comes to the tech options for the IVT reaction, Boulais says that neither of the two common approaches is fully optimized for mRNA.
“One approach is to use a bioreactor that’s usually used for cell cultures. But there are more sensors in there than what you need and it costs too much to run. Basically, it’s over-designed for the application,” she explains. “The other option is to use single-use technology (SUT) mixing bags, but here you might lack some sensors. So there’s a need for an in-between solution.”
Seeing the opportunity for innovations in the mRNA space, Boulais says that Sartorius is currently developing new products including an HPLC-based analytical solution to characterize batches at every step of the process and to maximize overall productivity.
Innovations in purification processes is something that Bill Whitford, Life Science Strategic Solutions Leader at DPS Group, says the industry sorely needs.
“Purifying mRNA in the lab is easy, and there are even kits to accomplish it,” Whitford says. “It’s in the large-scale purification where companies are having the most trouble. What they grabbed off the shelf for purification is working, but it’s not ideal.”
For example, an article2 published by the National Institutes of Health this year points out that the first published protocol for RNA production used size exclusion chromatography — but the method isn’t able to get rid of some impurities. Another method — ion exchange chromatography — can be used at scale to purify mRNA, but it is complex and requires additional equipment for temperature control. With the hopes of improving the process, manufacturers have also tried other methods — including oligo dT affinity, cellulose fiber, mixed mode resin chromatography and more
Yet, even with the experimentation, Whitford says he’s read that companies are suffering product losses of about 50% — a high rate in the industry — during the multiple purification and filtration steps.
Dealing with particular impurities is also an ongoing issue. For example, Whitford says that IVT generated trunked or double-stranded mRNA can emerge as a problem.
“Those are species you want to purify away from,” he explains. “Everyone has their own approach, but there’s not a perfect one.”
It’s also still unclear just how pure mRNA drug substance is supposed to be.
“They haven’t defined exactly what other impurity levels are a problem,” Whitford says. “So they might be over-purifying in some respects right now — and the cost of that is probably high because of the scale of manufacturing.”
Most likely, the large manufacturers that have jumped into the mRNA game for COVID scale-up have found purification processes that are working. But for now, those processes are proprietary and companies aren’t divulging the details. Looking ahead, the goal will be for the industry to develop a platform process that delivers the holy trifecta of being cost economical, fast and efficient at achieving a standardized level of purity.
Other challenges
Lipid nanoparticles and the cold chain
In the grand scheme of the world’s COVID-19 vaccination campaign, instances of dangerous side effects have remained fringe events. But for mRNA vaccines, some troubling reactions have emerged involving cases of two inflammatory heart conditions called myocarditis and pericarditis.
In July, analysis by the European Medicines Agency found that of 117 million administered Pfizer shots, 145 cases of myocarditis and 138 instances of pericarditis were reported. Most cases of the illness were mild — but five patients died (all of whom were either elderly or had preexisting heart problems).
Generally, the cause of myocarditis is often never found but it can sometimes be triggered by common viruses. As researchers work to uncover the link between the inflammatory conditions and mRNA vaccines, some are also casting a suspicious eye at lipid nanoparticles, the delivery system used for mRNA shots.
Researchers have already established that LNPs can have inflammatory properties.3 Now many are calling for a more systematic approach to uncovering the role they could play in triggering a host of inflammatory side effects from mRNA vaccines, including swelling, pain and the heart conditions.
Frustratingly, LNPs also have to be stored at frozen temperatures to ensure stability. With everyday pharma products, cold chain distribution and storage requirements are not a major issue. But in the context of a pandemic, it hampers the industry’s ability to rapidly and widely distribute vaccines.
Last year, Catalent took on a starring role in the efforts to manufacture vaccines, and struck a vial filling contract with Moderna. The U.S.-based contract manufacturer has since expanded that agreement into a long-term deal that could include support services for other mRNA products in Moderna’s pipeline. One of the chief issues Matt Blume, Catalent’s vice president of Business Transformation in Clinical Supply Services, says the company faced was ultracold storage and capacity, especially as mRNA products compete with cell and gene therapies for space on the cold chain distribution network.
“There has been a lot of demand for cold chain capacity in the last few years and that keeps growing,” Blume says.
To that end, Catalent has partnered with Stirling Ultracold, who has already supplied the company with 260 ultracold freezer units, and has plans to add more to the network.
With this increase in demand coming from all sides of the pharma industry, Masiella says that Stirling has had to grapple with longer lead times on delivering its freezer and delivery units.
“The toughest part for our customers was that typically, they would wait a week or two for their freezer, but during the pandemic, it was significantly longer,” Masiella says.
Orders for ultracold freezers are not likely to slow down any time soon and have instead shifted from North America and Europe to Asia, Africa and South America, as the entire world readies itself for mRNA. And in many places, the infrastructure for handling cold chain drugs still needs to be built in order for the distribution of mRNA vaccines and products to widen its reach.
Going forward, mRNA developers are looking at ways to tweak their drug — and potentially LNP — formulations so that they can be stored in more typical refrigerated temperatures and hopefully, also have a better side effect profile.
“The relationship between efficacy and side effects could potentially be improved with dose optimization, which could help cold chain storage as well,” Chancellor says.
Raw materials
With the recent explosion in cell and gene therapy investments, the market for plasmid DNA was already strained. Then came the sudden need for DNA in mRNA vaccine manufacturing.
In 2020, Aldevron, one of the world’s leading plasmid DNA manufacturers, estimated that over half of the world’s plasmid DNA capacity would be needed to produce 1 billion doses of an mRNA vaccine.4 In an effort to keep up, plasmid DNA producers have thus ramped up capacity to meet the surge in demand. But Boulais says that more pharma manufacturers could also opt to take matters into their own hands.
“We envision that mRNA manufacturers could integrate plasmid DNA manufacturing into their facilities,” she says. “At the moment, the industry is relying on third-party suppliers and we are expecting those processes to move in house.”
The enzymes needed for the IVT reaction are also a pain point in the industry. Not only are enzymes one of the most expensive components for manufacturers, they can also vary in quality.
“Raw materials suppliers are not in a regulated industry,” Atouf explains. “So the pharma company is responsible for ensuring the quality of that material.”
Inconsistency of materials between suppliers also remains an issue that forces pharma companies to invest significant amounts of time and money into testing their materials.
“High quality is one thing. But having a consistent raw material is a challenge,” Atouf says. “The moment you get a complex raw material you need much more additional testing and that’s where the questions are with mRNA.”
Expanding the supply chain of raw materials and improving quality and consistency will be a major linchpin to pharma making the process of mRNA production more cost-effective and efficient.
“The biggest risk with mRNA is with the supply chain for raw materials,” Boulais says. “Plasmid DNA and enzyme production will have to grow if we want to make this work.”
New horizons
The shift from using mRNA for COVID-19 to other critical vaccines and therapies is quickly picking up pace.
In June of last year, Sanofi Pasteur and Translate Bio, which specializes in mRNA, announced an expansion of a 2018 deal to co-develop mRNA vaccines for infectious diseases. Under the deal, Translate Bio could pocket nearly $2 billion, and the companies have already launched a phase 1 trial of an mRNA influenza vaccine. If the companies manage to develop a vaccine that’s more effective that the current shots for influenza, they could be tapping into a market nearly as big as COVID-19.
“The next large market that will be comparable to COVID will be influenza,” Chancellor says.
Yet, even though more mRNA products are headed into early trials, it will be years before they near commercialization. In the meantime, production for mRNA COVID vaccines is expected to hit its peak next year — setting up a potential capacity gap for mRNA manufacturing.
“At some point, the acute need to produce as many COVID vaccines will subside, so there will be a time when there is an excess manufacturing capacity for mRNA-type drugs,” Chancellor predicts. “But this capacity could be brought to bear on other diseases and challenges.”
It’s a situation that Stacey Treichler, associate director of Marketing, Biotherapeutics at Catalent, says the company is ready for.
“Because of how we’re set up in flexible suites that can handle different products, and because there are no special requirements for mRNA fill/finish capacity, we can adjust,” Treichler says.
As the mRNA market matures, the industry is likely to embark on several areas of improvement and take advantage of opportunities to generate more efficiencies.
A one-stop-shop?
As is the case with many pharma products, the value chain for mRNA vaccines is currently fragmented, with different steps of the process happening at various locations. Looking ahead, Treichler says that companies may try to move more parts of the processing steps under one roof.
“There are certainly scenarios where companies are good at one step, but not all the steps yet because it’s such a new process,” she says. “But I think companies will look at how they can become more integrated for customers so that they don’t have to negotiate with several partners when bringing products to market.”
Even if consolidation doesn’t happen, Atouf points out that drugmakers have found a way to make it work under the current conditions.
“It is possible for a company to achieve most steps in a single location. The question is: Why would you do that if the production is optimal in a fragmented landscape?” he says. “Besides, one of the learnings from the pandemic is that collaboration is so critical to overcome challenging manufacturing capacity situations.”
Self-amplifying mRNA
As companies look at ways to optimize dosing, one area generating a lot of buzz is self-amplifying mRNA.
Uğur Şahin, the CEO of BioNTech, the Germany-based company that co-developed the first-approved COVID-19 vaccine with Pfizer, recently explained the concept to Nature: “We are working in parallel on self-amplifying vaccines [in which the mRNA construct carries instructions to make copies of its antigen-coding sequence] and trans-amplifying RNA vaccines [in which one mRNA construct carries the antigen-coding sequence and another carries the instructions to make copies of the first],” he said in the June 2021 interview. “Each of these will have its own evolution.
I believe that the trans-amplifying technology could have quite a few additional advantages, including reduced dosing that could enable multivalent efficacy.”
If successful, Chancellor says that the innovation could be a great “enhancement” to the basic premise of mRNA technology.
“If you could use self-amplifying, you could get more bang for your buck with manufacturing capacity and a better-tolerated injection,” he says.
Downstream opportunities
For pharma manufacturing vendors, Whitford says there are plenty of opportunities to help drugmakers optimize the process of mRNA production with technology innovations.
“There are opportunities for Industry 4.0, such as AI and biosensors for process monitoring and digital twins for prediction and control… All of the facility of the future technologies could apply,” he says.
In particular, Whitford says that technology vendors could capitalize on the need for more efficient purification methods, and that specifically, new mixed mode resins — which filter impurities based on both size and chemical affinity — could be the jackpot.
“If you could devise a mixed mode resin that you pass your impure RNA through, and it came out pure because the resin had the right interactions and pulled out enough of the bad stuff, but the single-stranded RNA didn’t get touched… it’ll be a blockbuster,” he says.
Microfactories
Mini mobile factories have long been a dream in pharma as a way to more easily distribute needed medicines to remote parts of the world. And the technological challenge of this feat is so alluring that Elon Musk, CEO of Tesla, has hinted that his company could play a role in making it a reality.
Last year, Musk Tweeted that Tesla had collaborated with CureVac, a German company developing a COVID-19 vaccine, on an RNA bioreactor and that the pair could have a microfactory in the works.
“In principle, I think synthetic RNA (and DNA) has amazing potential. This basically makes the solution to many diseases a software problem,” he said on Twitter, following it up with: “Tesla, as a side project, is building RNA microfactories for CureVac & possibly others.”
Although no further details of a collaboration between the companies has been released, increased interest and investment from automation experts like Tesla are likely to be a boon for improving mRNA manufacturing in traditional pharma plants — or microfacilities of the future.
“A factory-in-a-box, prefabricated modular container housing a vaccine manufacturing plant could be turned on a week after delivery, providing all but the fill/finish for even the smallest countries to start making mRNA vaccines,” Whitford says. “That’s really desirable in a pandemic response.”
Next-gen mRNA
As the industry looks ahead, it’s tough to say how long these innovations — large and small — will take. But without a doubt, new mRNA therapies and vaccines are on the way.
“The first RNA drugs were in development over 20 years ago,” Chancellor says. “But now, the massive investment means that there’s not going to be a long tail between new therapies. Whether it’s going to be five, 10 or two years until there are new mRNA products, we don’t know. But once they come, they will come fast.”
And as companies prepare to bring new mRNA products online, process innovations will happen in tandem.
“Given the situation with COVID, companies had to go fast,” Boulais says. “There were two things that could not be compromised: safety and efficacy. Now, issues like formulations and storage that have not been ideal will be improved in the second generation processes.”
References
- Verdecia, Mark; Kokai-Kun, John; Kibbey, Maura; Acharya, Sarita; Venema Jaap; Atouf, Fouad. “COVID-19 vaccine platforms: Delivering on a promise?” Human Vaccines and Immunotherapies. Published May 25, 2021.
- Rosa, Sara Sousa; Prazeres, Duarte M.F.; Azevedo, Ana M.; Marques, Marco P.C. “mRNA vaccines manufacturing; Challenges and bottlenecks.” U.S. National Library of Medicine, National Institutes of Health. Published March 24, 2021.
- Dwivedi, Ramya. “Research looks at inflammatory nature of lipid nanoparticle component in mRNA vaccines.” News-Medical.net. March 15, 2021.
- Raper, Vivienne. “Plasmid manufacturer scales up production capacity to serve new therapeutics.” Genetic Engineering and Biotechnology News. Aug. 11, 2020.