It’s no secret that drug delivery and packaging is a vital part of product manufacturing and commercialization. And making the right delivery choice for a new drug can help make or break that product’s success.
Regulatory scrutiny of drug delivery and packaging products has also increased in recent years in step with the rise of sterile processing — putting even more pressure on vendors to innovate materials and containers that protect drug safety and efficacy.
“Over the past several decades the biopharmaceutical industry has produced an impressive array of advances,” says Ron Verkleeren, vice president and general manager of Corning Pharmaceutical Technologies. “At the same time, the standards for quality and safety have continued to increase steadily, driven by the same societal factors that affect other industries.”
Corning’s Valor Glass was designed to improve manufacturing throughput on both new and older filling lines.
Here’s a look at some of the ways drug delivery and packaging vendors are innovating solutions to meet the industry’s emerging challenges.
Targeted delivery
One of the growing trends in pharma has been to put more focus on the patient experience, which has been a key driver in the development of drug delivery products as well.
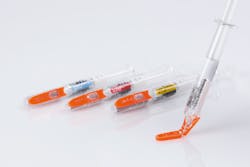
“When it comes to unit-dose medications for injectables, there is a growing trend towards pre-filled syringes. They make injections easier and safer for healthcare personnel as well as for patients in a growing home care market,” says Gene Dul, president of Schreiner MediPharm U.S.
Dosing with pre-filled syringes not only offers greater precision and decreases the risk of overdosing, these kinds of syringes can often be enhanced with additional features, such as multi-functional labels.
“For instance, label-integrated features can include detachable parts to document drug administration in the patient’s medical records, gradation lines for safe dosing, authentication technologies for anti-counterfeiting protection or temperature indicators that show the right injection temperature,” Dul says.
With those goals in mind, Schreiner MediPharm developed its Needle-Trap protection labels, which secure the needle after the injection has been performed, and are now being used with Pfizer’s epoetin biosimilar, Retacrit.
Martina Christiansen, head of Sales and Marketing Pharma of Hoffmann Neopac, says that an increase in the use of high-potency medicines has also led to a greater need for quick and targeted drug delivery devices. In particular, Christiansen notes that “highly potent medicines are requiring exacting doses, making single-dose packages more attractive to prevent over- or under-use.”
Schreiner MediPharm’s Needle-Trap features a plastic needle trap which is an integral component of the label for prefilled syringes, and serves to secure the needle after the injection has been performed.
Because “these finicky formulas are highly sensitive to moisture, oxygen or light (or all three),”
Christiansen says that Neopac’s Polyfoil tube solutions come with a cap that is irrevocably opened once it’s twisted off. The solution includes a proprietary blend of materials that helps protect the product against external threats.
“In addition to tamper-evidence, the tube’s permanently affixed cap emphasizes consumer simplicity and offers accurate application of the enclosed product to the area of treatment,” she says.
Quality control
“Modern drug products demand higher levels of quality in their packaging components, and regulatory guidelines have continued to raise the bar on acceptable quality over time,” says Erin O’Brien, vice president, Product Management and Marketing Operations, West Pharmaceutical Services, Inc.
According to O’Brien, the emphasis in the 2000s was on extractables and leachables, and harmonizing global testing standards for container closures. Today, developments such as USP <790>, USP <382>, and FDA Combination Product Guidances have shifted the focus towards the expectations around particulates and cleanliness, as well as the performance of closures.
To that end, O’Brien says that West has continued to upgrade its most modern elastomer formulations to improve quality standards and include enhancements such as FluroTec barrier films, B2-coating and value-added post processing of elastomeric components.
West also recently launched its Westar Select component line, a high-quality offering for pharmaceutical wash and sterilized products, comprising a defined global product portfolio focused on component capability with a tighter visible particulate specification.
As Verkleeren notes, rising regulatory scrutiny has also prompted packaging vendors to develop safer materials for drug storage and delivery. According to Verkleeren, Corning’s Valor Glass line was “developed specifically to address these higher performance requirements that are essential to enhancing quality for patients and improving pharmaceutical manufacturing.”
Because Corning’s Valor Glass line can eliminate damage or breakage, it can reduce particulate contamination and prevent cracked containers, which may otherwise lead to a loss of drug product sterility.
“These are some of the leading causes for drug product recalls and pharmaceutical manufacturing issues,” says Verkleeren. “In addition, Valor Glass has consistently demonstrated improved manufacturing throughput on both new and older filling lines.”
Just as pharma companies have developed cutting-edge treatments for a range of diseases, drug delivery vendors are rising to the challenge of delivering those drugs as safety and effectively as possible.