Biopharma manufacturers know that adoptive cell therapies (ACTs) such as chimeric antigen receptor (CAR) T-cell therapies are unlike anything that has come before.
ACTs are living drugs. Specifically, they’re human cells that, when modified or expanded, gain efficacy in the treatment of cancer. CAR T-cells in particular have shown unprecedented success in the clinic and they are bound to bring much-needed relief to thousands of people with cancer in the years to come. However, increasing demand for ACTs will come with a critical need for a new approach to biopharmaceutical manufacturing. Unlike small molecule and protein-based drugs, whose discovery process is relatively standardized and whose manufacture is stoichiometrically predictable, ACT development and manufacturing involves multiple layers of variability and complexity.
CAR T-cells have progressed far into the clinic despite these manufacturing limitations: Patients in the U.S. with different types of blood cancer can receive CAR T-cell therapy in over 150 treatment centers in 38 states and the District of Columbia. The fact that CAR T-cells reached the clinic at all is a feat of biomedical engineering. Still, CAR T-cells are not the end of the story for ACT development. Several other types of ACT are under investigation and in the next decade or so, CAR T-cells might find company in their space.
Other ACTs on their way to the clinic include T-cell receptor (TCR) T-cell therapies, tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells. This burgeoning pipeline offers hope to people with cancer who do not have access to safe and effective treatments.
The growing popularity of ACTs also cements the reality that the manufacturing process for cancer treatments will never be the same. This article will explore the manufacturing challenges that still impact CAR T-cell developers and the hurdles that developers must still overcome as other types of ACTs speed towards clinical use.
CAR-T manufacturing challenges
CAR T-cells are custom-designed and specially constructed immune cells that selectively target tumor cells for destruction. T-cells normally kill foreign invaders in the body, but tumor cells typically evade the body’s defenses and grow unrestrained. By transfecting T-cells with the gene for the CAR protein, T-cells can be trained to identify cancer and destroy it as if it were any other foreign invader.
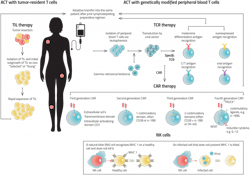
Today’s CAR T-cells are autologous, meaning that each therapy is derived from an individual patient’s blood. After a patient has their blood drawn, the samples are sent to a CAR T-cell manufacturer, where the manufacturer isolates the T-cells and engineers them to express the CAR gene using a virus or transposon system. The cells are then expanded and returned to the clinic, where a physician reinfuses the cells into the patient’s bloodstream.
These cells’ autologous nature creates benefits and drawbacks for both patients and manufacturers. Since the cells come from a patient’s own body, the chances of eliciting an adverse immune reaction based on incompatibility are slim. On the downside, autologous CAR T-cell production is slow; it can take several weeks for the treatment to arrive at the clinic. Several stepsmust take place before manufacturing can begin: The patient must be diagnosed, a physician must decide to pursue CAR T-cell therapy, and the patient’s blood must be drawn and sent to the lab. Only then can the manufacturer engineer the cells to treat cancer. Every day that a patient must wait for the treatment to arrive is one less day of receiving a treatment that may save their life.Biopharmaceutical companies are actively seeking ways to develop allogeneic CAR T-cells. These cells could drastically reduce wait times by offering a standardized, off-the-shelf treatment option. However, since these cells are foreign to the body, they pose additional risks. For example, the patient’s immune system may kill the cells before they have any therapeutic benefit, or worse, the infusion can cause graft-vs-host disease, where the CAR T-cells attack healthy tissue.
Regardless of their source, CAR T-cells pose several manufacturing challenges. Since their development cannot be completely controlled, the safety and efficacy of any single batch cannot be guaranteed. For example, when introducing the CAR gene to T-cells, the transfection step is not perfectly efficient. Some cells may not accept the transgene and never become CAR T-cells. On the other hand, too many copies of the transgene may integrate into each genome, posing a potential safety issue.
If a cell contains more than four copies of the transgene, the cell can become toxic and cause an inflammatory response that leads to organ dysfunction and death. Consequently, if a batch contains this many copies per cell, the Food and Drug Administration recommends that manufacturers screen it out. Finally, some viral vectors used for transfection may make it through the filter steps and end up in the final batch. If these viruses are replication-competent, they could theoretically cause the growth of T-cell neoplasms, another form of cancer. For these reasons and more, manufacturers need to use quality control methods to screen out suboptimal batches before they reach patients.
Fortunately, the tools and techniques needed to monitor the quality of CAR T-cell therapies already exist. Techniques such as flow cytometry and Droplet Digital PCR (ddPCR) can be used to monitor transfection efficiency, CAR-T copy number and the presence of replication-competent viruses and other contaminants.
Currently, researchers are developing novel CAR T-cell therapies, as well as other ACTs, that overcome several of these limitations.
Treating solid tumors with TCR T-cells
One drawback of CAR T-cells is their low efficacy in treating cancers beyond those circulating through the blood. These cells are limited by their relative inability to infiltrate solid tumors (such as those found in breast or prostate tissue). But T-cell receptor T-cells may help solve this challenge.
Like CAR T-cell therapy, TCR therapy involves genetically modifying T-cells to express a protein that targets tumor cells. But unlike CAR T-cells, which only target cells that express certain cell surface proteins, TCRs can direct T-cells towards any antigen, whether it is expressed on the surface of a tumor cell or internally.4 A fragment of that antigen simply needs to be presented by a tumor cell’s major histocompatibility complex (MHC).
MHCs are protein structures that help T-cells differentiate between types of cells. TCRs recognize the MHCs on tumor cells as foreign and then signal T-cells to destroy the cells. In other words, TCR T-cells amplify the adaptive immune response to tumor cells. Since TCR T-cells can identify foreign cells by their intracellular antigens, they could potentially be effective in treating solid tumors.
TCR T-cell manufacturing poses similar challenges to CAR T-cell manufacturing. Manufacturers must still be careful to monitor cells for transfection efficiency and copy number, as well as the presence of harmful contaminants. Also, like today’s CAR T-cells, the TCR T-cells currently under development are autologous and therefore take weeks to manufacture, creating similar delays and risks for patients. An allogeneic TCR T-cell could theoretically reduce wait times and increase the efficacy of this promising therapy.
Boosting innate immune activity with TILs
A tumor-infiltrating lymphocyte (TIL) is any T-cell or B-cell that has successfully entered a tumor mass. These cells’ unique ability to infiltrate solid tumors gives them immense therapeutic potential. It also tells us a lot about how these cells could function in the clinic.
For instance, TILs naturally recognize tumor antigens and do not need to be genetically modified. Rather, to be used as an autologous therapy, they merely need to be made stronger. This involves taking a blood sample, isolating and expanding the TIL cells and returning them to the patient.
NK cells: A different kind of lymphocyte
Not all ACTs rely upon T-cell activity. For example, natural killer cells are another type of lymphocyte that evolved specifically to attack tumor cells. These cells’ unique mechanism of action gives them potential in the clinic and might make the manufacturing process simpler and cheaper.
When NK cells identify a tumor cell, they attack the tumor and release chemical signals that activate the adaptive immune system against it. Since these cells seek out tumor cells on their own, they do not need to be genetically modified to be used as cancer therapy, making the discovery and manufacturing process much simpler.
NK cells are considered safe, even when they are allogeneic. This characteristic makes them a suitable source of off-the-shelf ACTs. With this flexibility, manufacturers can save time and money by manufacturing larger batches. This pays off for patients, as well, who will not have to wait weeks to receive ACT.
Despite their natural tendency to attack tumors, NK cells cannot overcome tumors without help because the tumor microenvironment suppresses their activity. Here, the CAR protein may be able to help. Engineering NK cells to express the CAR protein may boost their activity and help them overcome the immunosuppressive behavior of tumor cells.
NK cell manufacturing is not without its challenges. NK cells are difficult to expand in vitro. Also, in most cases, NK cells do not infiltrate solid tumors, limiting their potential in treating cancers beyond blood cancer. However, as a treatment for leukemia and lymphoma, NK cells promise a more effective, easier-to-produce ACT option.
Adoptive cell therapy’s next act
The FDA approval of CAR T-cell therapy was only the beginning for ACTs. As TCR T-cells, TILs and NK cells reach the approval stage, they may all one day offer hope to patients with a wider variety of cancers.
As manufacturers aim to produce safe and effective therapies quickly and inexpensively, all of these therapies will create new challenges. But biomanufacturers do not have to face these challenges alone: regulators believe in these therapies so much that the FDA has published several guidances aimed at helping biomanufacturers improve their ACT manufacturing practices.
With support from regulators and unrelenting demand from patients and physicians, biomanufacturers will hopefully discover new ways to make ACT manufacturing more efficient so more treatments can reach more patients while they can still make a difference.