Since the approval of Zoladex and Lupron Depot for the treatment of prostate cancer in 1989, the use of polylactide (PLA)/poly lactic-co-glycolic acid (PLGA)-based delivery systems has enabled the development of extended-release formulations that can reduce dosing frequency and minimize drug side effects.
For example, Risperdal Consta, the first long-acting injectable atypical antipsychotic approved by the U.S. Food and Drug Administration and indicated for the maintenance treatment of bipolar I disorder as monotherapy, has been shown to provide superior efficacy compared with oral atypical antipsychotics, with a low level of treatment-emergent side effects and favorable patient acceptance. Its prominent role in the treatment paradigm is largely attributed to improved compliance with treatment derived from the administration of the long-acting, injectable PLGA microsphere formulation every two weeks in a physician’s office — compared with self-administration of the oral formulation on a daily or every-other-day basis.
PLA/PLGA microspheres have been incorporated into a multitude of pharmaceutical products used for the treatment of a wide array of indications, including cancer, psychiatric disorders, endocrine disorders and periodontal disease. In fact, to date, the FDA has approved 16 PLA/PLGA-based products, of which 12 are formulated as microspheres. Many of these products have become leading medicines in their respective categories due to enhanced safety, efficacy and dosing profiles.
Despite this market potential, there are no generic versions of PLA/PLGA products, even for those products for which patent protection has expired. This highlights the inherent complexity of manufacturing a PLA/PLGA-microsphere product under the reproducible and exacting standards of the Good Manufacturing Practices (GMP) that are required to achieve FDA approval while also meeting desired parameters for drug loading, dose-release profiles, route of administration and cost-effective drug development.
The Complexity of Manufacturing PLGA Microspheres
The complex and proprietary nature of the biologics manufacturing process creates a high bar when it comes to the development of biosimilars, because even a small change to the process can have a substantial impact on the safety, efficacy, activity or stability of the final product.
As a result, although 18 biologics are off patent, only seven of these products have FDA-approved biosimilars. Products with multi-billion-dollar U.S. sales in 2017, such as Rituxan, Xolair, and Tysabri have no generic competition despite the absence of patent protection and the large market.
The complexity of manufacturing PLA/PLGA microspheres creates a similar challenge when planning to develop new extended-release therapies or generic versions of branded products. The challenges stem from several factors, including local drug diffusion and polymer degradation in vivo. PLA/PLGA begins to degrade following administration, resulting in an increase in the amount of water-accessible space leading to the interior of the particle, and accumulation of lactic and glycolic acid that may substantially lower the pH within the interior of the particle. The decreased pH can further accelerate particle degradation, and the composition of the polymer and its inactive ingredients have a significant impact on the time-release properties of the product.
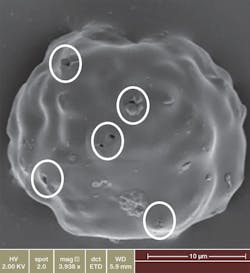
Representative scanning electron image of a Zilretta microsphere showing surface characteristics as well as channels for drug release.
The challenges of manufacturing a PLGA microsphere in particular are highlighted by a study in which multiple formulations of microparticles containing risperidone yielded statistically significant differences in drug loading. This study also highlighted the challenge in finding manufacturing assays that even enable comparative evaluation of in vitro drug release profiles for establishing equivalence among various microsphere formulations. The extent to which the complexity of PLA/PLGA manufacturing raises the bar for the development of generic forms of branded PLA/PLGA products is underscored by the fact that the FDA’s Office of Generic Drugs has awarded a variety of grants and contracts to support research in five areas that are critical for expanding development of PLA/PLGA-based drug products: development of in vitro-in vivo correlations (IVIVC); development of in vitro release testing (IVRT) methods; understanding of the impact of properties of PLGA polymers on product performance; modeling and simulation of PLA/PLGA-based drug products; and investigating potential peptide PLGA interactions during product manufacturing and use.
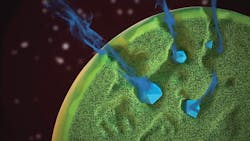
Drug release from Zilretta microspheres demonstrating the mechanism of release.
Despite these challenges, innovation in PLGA manufacturing continues. In October 2017, the FDA approved the 16th PLGA microsphere product, Zilretta, for the treatment of osteoarthritis (OA) knee pain. This approval demonstrates that adapting PLGA manufacturing methods to meet the clinical needs of specific disease indications can be a powerful driver for bringing new therapies to market.
The following case study outlines the design and manufacturing approach undertaken to leverage PLGA microsphere technology into a new treatment option for patients with OA knee pain.
A Case Study in Innovation
Zilretta was developed by Flexion Therapeutics, Inc., a biopharmaceutical company in Massachusetts, as a novel PLGA formulation that was designed to enable a long-acting, non-opioid treatment for OA knee pain. The FDA approved Zilretta as an intra-articular injection for the management of OA pain of the knee. Flexion’s approach to developing Zilretta serves as an example of how the design and manufacturing of a microsphere formulation can be tailored to meet the clinical needs of a specific patient population.
In developing an extended-release therapy for the treatment of knee osteoarthritis, Flexion was seeking to address an unmet clinical need for patients living with this chronic painful condition. Non-opioid therapies include the injection of immediate-release (IR) corticosteroids, such as triamcinolone acetonide crystalline suspension (TAcs). While the analgesic effect of immediate-release steroid injections typically lasts two to four weeks, by medical convention, these drugs are not administered any more frequently than once every three months.
We set out to develop a TA formulation that enabled an extended-release dose of the medicine at the source of a patient’s OA pain, and could provide pain relief for the entire duration of the dosing period. While other therapies administered by intra-articular injection to the knee rapidly leave the joint, we sought to develop a formulation that would remain in the knee joint to optimize persistent drug concentrations within the target tissue while minimizing systemic exposure to TA. This is an important consideration when delivering a drug that could potentially have off-target effects if administered systemically, as such effects can have a negative impact on safety profiles and are associated with product development failure. Based on the success of currently approved PLGA-based products that provide sustained efficacy, Flexion chose to develop a PLGA formulation of TA for the treatment of knee OA pain.
Thinking Outside the Box
[pullquote]Before creating a new PLGA microsphere formulation, Flexion had to start by addressing the limitations of existing PLGA technologies, such as the typical emulsion-based process used to commercialize a majority of the approved microspheres. First, these technologies can exhibit a high burst release, which means that a large fraction of the dose is immediately released upon injection. This may result in excessive drug concentrations at the administration site while reducing the amount of drug within the microsphere that is delivered over a longer period of time.
Second, the manufacturing process needed to be completely sterile. And third, every active pharmaceutical ingredient has a different set of attributes that needed to be taken into consideration in the PLGA formulation. In other words, each PLGA formulation is a completely customized product based on the drug being delivered, the desired time-release profile and the location and route of administration.
Rather than adapting the profile of a new drug to the constraints of current PLGA technologies, Flexion took a step back and identified a set of defined clinical objectives that the PLGA product needed to achieve: (1) an extended-release to provide sustained pain relief following a single dose; (2) a quick onset of action but a low burst after injection to minimize systemic exposure to drug; (3) ease of administration; (4) simple packaging into the volume needed for an intra-articular injection.
With these objectives in mind, the team identified and implemented innovative PLGA manufacturing processes. First, they used a scalable, non-aqueous process that allowed the product to be terminally sterilized by irradiation. This enables a high level of sterility assurance while maintaining cost-effective manufacturing.
Second, the team developed a formulation with a release mechanism that would maintain concentrations of the drug in the knee joint for at least 12 weeks. Release mechanisms differ depending on the route of administration, and may vary across intramuscular, subcutaneous or intra-articular injection. Therefore, it’s important to understand the desired release profile of the product, as well as the way in which the route of administration will affect this profile. Environmental and physical conditions of local delivery are critical factors to consider when designing a microsphere product, and the structure of the knee joint requires a different set of design criteria compared with other routes of administration due to the higher volume of drug injected into the knee compared with other sites. Our formulation took these factors into account and resulted in a novel product with an extended-release profile optimized for intra-articular injection that met the company’s product design objectives: prolonged residence within the knee joint, long duration of action, and minimal systemic side effects.
Scale Matters
Scalability is essential to the success of any new manufacturing process. The team designed scale-up processes for Flexion’s proprietary PLGA microsphere technology when Zilretta was still in Phase 2 clinical development. This allowed larger amounts of product to be manufactured by increasing the run time of the existing process — rather than requiring the development of a new process that could operate at a higher scale. The ability to retain the same process eliminated development and regulatory risks that might have resulted from changes to equipment or critical aspects of the process. This allowed Flexion to submit a package on manufacturing and product stability to the FDA based on Phase III product manufacturing. They were then well-positioned for a full commercial launch within weeks of the FDA approving Zilretta — a significant accomplishment for a small company launching its first product.
Commercial-scale manufacturing for Zilretta was established at Patheon, a contract manufacturing organization. Flexion chose Patheon, a Thermo Fisher company, for its “condo model” in which each client company has a dedicated suite for manufacturing. Flexion retains control of the manufacturing process knowledge while the CMO controls the GMP environment in Flexion’s suite.
This type of arrangement gives Flexion access to a commercial-scale infrastructure without having to invest in a proprietary manufacturing facility, while enabling the company to maintain control over manufacturing cadence. It avoids the potential for scheduling challenges that often exist with traditional CMOs that routinely require manufacturing runs to be booked six to 12 months in advance. This type of model could be especially attractive to companies developing innovative technologies that may not easily fit into the existing manufacturing process that CMOs typically offer.
Creating Clinical Value with PLGA
Scientific and technologic discovery is a critical driver for improving clinical care. Flexion’s experience with Zilretta underscores how critical drug delivery innovation is, and highlights how continued advances in the development of novel PLGA microsphere formulations can provide significant clinical benefits. The ability to innovate additional microsphere formulations could facilitate the development of new classes of therapies that hold the potential to address real clinical need, especially in chronic diseases that require long-term treatment (i.e. OA or autoimmune diseases). Continued development of novel drug formulations and drug delivery approaches will play a critical role in catalyzing therapeutic advances that improve patient care and outcomes. Realizing the clinical potential of PLGA microsphere technology requires that the pharmaceutical industry rise to — and overcome — the real but solvable challenges of PLGA product design and manufacturing.
[javascriptSnippet]