Improving Digital Quality in the Pharmaceutical Industry
Digital in general and Artificial Intelligence (AI) specifically is one of the transformational technologies in the next decade. It has already transformed many industries and functions. Bitcoin and driverless cars are often touted as the most advanced forms of digital in practice already. However, there are many other examples in place already such as Siri, Google Translate, the iRobot Roomba vacuum cleaner, Surveillance, etc. All these examples use AI in various forms. Within the healthcare industry, AI is now in use to augment the physicians in diagnosing cancer and reading x-rays & medical images. Certainly, these are but the first of many breakthroughs.
In our travels, it is clear that there is no common vision for the role of digital and AI to improve quality within the pharmaceutical industry. Indeed, there is probably a lack of understanding on what it means and what the potential opportunities are. This article will describe a vision on how digital could transform quality within the pharmaceutical industries and describe various use cases. First, we need to define quality, digital and AI.
Quality: Today, many within the industry think of the terms quality and compliance as interchangeable. At PwC, we, however, believe that quality is much more than compliance and indeed more focused on product quality and should be defined from a patient perspective (see figure 1). One definition is “The availability of robust medicines and reliable devices manufactured in a highly predictable and compliant manner that improve the quality-of-life for the patient.” Based on this definition, it is clear that quality is driven by the patient, as well as the design of the product & manufacturing processes, manufacturing operations, and the supply chain functions. From this perspective, the quality organization is an enabler of quality, but development, engineering, technical operations, manufacturing, etc. have a much larger and direct impact on quality. This discussion will focus on the broad definition of quality, not just quality as practiced today by the quality organization.
Digital: Figure 2 below highlights a few of the many components of digital and are certain to evolve over time. It includes hardware (drones, robotics, 3D printers, wearables, mobile devices, smart sensors, Internet of Things, location detection), software (Cloud computing, Big Data analytics, blockchain, Artificial Intelligence (AI), Robotic Process Analytics (RPA), visual recognition, virtual reality, augmented reality).
Artificial Intelligence: AI is a subset of digital, but it is important enough to call out separately. AI is a very general term and by itself not very useful. There are many distinct components of AI that are useful and implementable that can impact quality. Key components of AI are: voice recognition, computer vision systems, Natural Language Processing (NLP), machine learning, deep learning, predictive analytics, robotic process automation (RPA), and report writing.
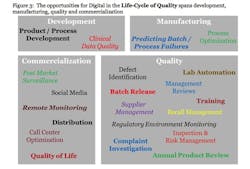
THE LIFE CYCLE OF DIGITAL QUALITY
Quality begins in the development phase through designing, formulating, and/or testing with the purpose of developing robust medicines and reliable devices and associated manufacturing processes. After a technology transfer, manufacturing has the challenge of assuring the availability of highly predictable products. The quality teams ensure the products are manufactured in a compliant manner. Ultimately, during commercialization, the company must ensure the products are improving the quality-of-life for the patients. The blue initialized words link directly to the definition of quality above and demonstrate that quality is truly a cross-functional effort. Use cases are described below for each of the four phases of the lifecycle.
Development: During the Development Phase, AI can be used in 2 use cases related to improving quality.
1) Product / Process Development: AI can be used to predict optimal product designs (formulations, specifications, manufacturing processes, test methods, etc.) through leveraging concepts such as Knowledge Management (KM) and Quality by Design (QbD). AI can be used to extract knowledge from all previous development efforts as well as manufacturing data. Although KM and QbD have been promoted over many years, technology is now at a point where this is feasible. For example, PwC’s BodylogicalTM is a peer reviewed computational model of a human body that is able to simulate the outcome of health interventions on individuals. It was built after years of extensive research and coding of how the body works and is now able to forecast results for an individual patient. Pharmaceutical companies can use BodylogicalTM to model how the body reacts to therapies, thereby creating possibilities for faster, better targeted and less expensive clinical trials, accelerating time to market and improving health outcomes.
2) Clinical Data Management Quality: While there are many use cases for AI in the clinic, this use case is specific to the data Quality aspects. AI can be used to monitor the completeness, accuracy, and timeliness of the data as well as identify trends across data points to highlight potential issues with data integrity. Ultimately, it can correlate the clinical outcomes back to the manufacturing and QMS related data. As an example, it will be possible to monitor clinical outcomes of insulin usage through real time diagnostics and correlate the outcomes directly back to the individual lot of insulin used. This is the ultimate in transforming quality from a compliance focus to a patient-outcome focused activity.
Manufacturing: Within manufacturing, AI can also be divided into 2 broad use cases.
3) Process Optimization: Analytics can be used to drive insights into the massive amounts of process data and correlating it to the output (quality, clinical, etc.) data to understand the critical process parameters. This in turn can lead to optimizing the process parameters, improving process capability and ultimately improving the yield profile. The data generated by Manufacturing Execution Systems (MES) and electronic batch records are fundamental to process optimization.
4) Predicting Batch/Process Failures: Through ongoing, real time monitoring of process parameters through IOT-based sensors, it is possible to identify potential failures and adjust the processes accordingly. This can reduce the number of manufacturing related non-conformances significantly.
Quality: There are at least 11 use cases for the quality phase and potentially many more.
5) Defect Identification: Potential defects can be identified using visualization algorithms developed by machine learning techniques on high speed manufacturing lines such as tableting, liquid vial filling and device assembly lines.
6) Lab Automation: Lab automation deserves a dedicated white paper; however, it is worthwhile noting that the data generated through the lab is an integral part of any Digital strategy for quality. The outcome data (test results) are critical in gaining the understanding of the development and manufacturing processes (use cases 1, 3 & 4).
7) Batch Release: Real time batch release is a worthwhile objective; potentially the holy grail of manufacturing. It can significantly reduce cycle time and reduce costs. Using parametric release based on the real time process monitoring mentioned above can provide the basis to realizing the this goal. Blockchain platforms and smart contracts could be utilized for similar real-time monitoring and batch release of products manufactured by contract manufacturers (CMOs).
8) Training: Augmented and Virtual Reality (AR/VR) can be used to enhance the training experience of operators. This coupled with electronic SOPs and Work Instructions can reduce human error during manufacturing and testing as well as providing on-the-job training guides.
9) Regulatory Environment Monitoring: The pharma and medical device industry is heavily regulated. Programs can be developed to monitor regulatory trends on a global basis and even highlight which changes might have an impact on your company’s product portfolio.
10) Inspection and Risk Management: Analytics can be used to automate part of the Internal Audit processes. It can highlight potential areas of risk for further deep dive. For example, it can spot insufficient depth in investigations into the root causes of complaints and non-conformances. Analytics can also generate heat maps of potential risk areas through trending and reporting metrics for use in management reviews.
11) Supplier Management: There are already services that provide insight to the global supplier base and can provide (political, regulatory, geographic, financial) risk assessments. These services can be augmented by analytics related to supplier quality and correlated with process, product and patient results.
12) Complaint Investigations: Complaint investigations can be (partially) automated through algorithms to analyze all relevant data (batch records, lab results, previous complaints, scientific literature, etc.) to provide guidance rapidly closing complaints and or conducing avital in-depth analysis.
13) Recall Management: Analytics can be a major benefit in streamlining the recall process in understanding the impact of recalls and speeding up the process.
14) Annual Product Review (APR): Ongoing process validation linked with post market surveillance (mentioned below) can become the basis for automating the APR process. Not only can trends be analyzed real time, graphs and even the final report can be generated automatically. Programs and algorithms already exist that automate the writing of news stories, especially in the financial sector.
15) Management Reviews: Provide real time insights on the data & trends for use in the management reviews on key product and process quality issues.
Commercialization: Digital is already having a large impact on the commercial landscape, these use cases are from the quality perspective.
16) Post Market Surveillance: Processing complaints using NLP to determine serious adverse events (SAE) and medical device reportables (MDR). Reports can be generated automatically and filed with the various agencies with minimal, if any, human interactions.
17) Social Media: Provide real-time sentiment analysis monitoring of various social media sites to highlight potential quality issues
18) Remote Monitoring: IoT capabilities are already providing major advances in monitoring and servicing equipment in the field. But this is not limited to capital equipment, it also relates to the smart pill in monitoring patient compliance with the prescriptions
19) Distribution: There has already been much publicity on using drones to deliver medicines in critical situations in both emerging and developed markets. However, digital can contribute in other areas as well. IoT can improve the monitoring and quality of the overall logistics and cold chain. Blockchain will be a major enabler of track & trace, ePedigree and anti-counterfeiting capabilities.
20) Call Center Optimization: Customer complaints can be transcribed from voice to text in real time in a call center to capture customer complaints as well as the use of sentiment analysis tools to provide guidance to the operator for the appropriate questions and tone of voice to use to provide a pleasant experience to the patients and caregivers.
21) Quality of Life: Under the premise that quality is ultimately defined by the patient. E.g.: the improvement in the quality of life for the patient, the ultimate role of digital (wearables, IoT, Apps, etc.) is to monitor patient related physiological data real time and link it back to specific manufacturing batches.
IMPLICATIONS OF DIGITAL QUALITY
Digital quality has many implications across the organization. Foremost, data scientists will become critical in developing the AI algorithms and managing the data. Engineers across development, operations and quality will need to work as equals on a cross-functional team to improve quality across the life cycle.
AI relies on ‘big data’. The AI algorithms become exponentially more accurate as more data becomes available. A major challenge will be to assimilate the current data and use it to train the algorithms.
In addition, regulators will also need to become comfortable with AI. AI is inherently un-validatable in the traditional sense of validation. New rules and techniques will need to be defined in coordination with regulatory agencies and industry.
BENEFITS OF DIGITAL QUALITY
Digital quality will leverage the collective knowledge of the organization and expand it dramatically. It will allow for the faster development of new medicines as well as improve the speed to market. On the manufacturing side, it can predict & prevent failures, increase yields and improve quality.
This article has provided an overview of the technologies in order to de-mystify the digital landscape and highlights the various use cases of digital in driving quality throughout the product lifecycle. These use cases will without doubt evolve over time as companies develop experience and uncover new opportunities. In our opinion, digital will not replace humans, but instead augment the human capability. Combined, humans and digital will deliver higher quality products than either humans or digital alone can deliver.
Some companies are already benefiting from their digital journey through improved yields in manufacturing, complaint management and other areas. However, each company has different strategic objectives and a different starting point for their digital quality journey; hence, there is no simple one-size-fits-all solution. A key requirement for anything AI is having a robust data set; hence, this could be the driver for defining the starting point for your journey.
What is your digital journey? What is your data strategy? Do you have active programs in place? Do you have the right capabilities? If not, it is time to get started —your competitors are already doing so.