Lifecycle Management Strategies Can Uncover Hidden Value
The importance of a solid lifecycle management strategy has been long acknowledged in the pharmaceutical industry. However, more emphasis should be put on the entire pharmaceutical product lifecycle — from discovery through the end of life, not only from launch to patent expiry. The entire network of stakeholders including governments, payers, physicians and patients increasingly requires more value for their investments in new therapies. As Marko Salo, vice president, marketing and sales, for API manufacturer Fermion Oy, puts it, “Value through lifecycle management can be demonstrated by improving efficacy, reducing side effects, simplifying dosing and increasing patient compliance.”
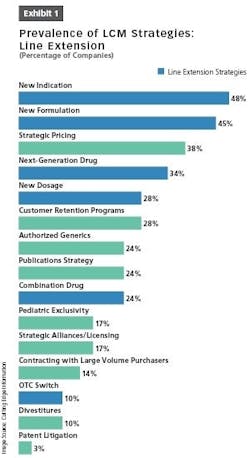
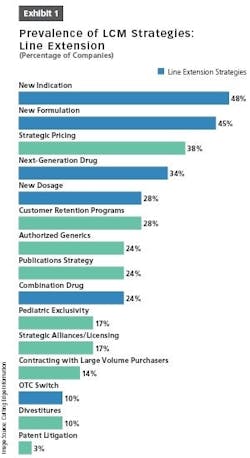
Other opportunities afforded by LCM strategies include finding new delivery devices and packaging solutions; more efficient logistics; improved formulations; and improved manufacturing processes aimed at optimizing batch sizes, shortening process lead times and making the process more robust (Exhibit 1).
“As an API CDMO, lifecycle management for Fermion often means post-launch R&D activities aimed at quality and cost improvements that lengthen the client’s product lifetime, the client business relationship lifetime, and grow the business,” says Salo. “When planning the product development strategy with our clients, we consider LCM aspects early on in product development. Pharma companies can utilize the LCM expertise of CDMOs best if they start collaborating with CDMOs from the early discovery stage through to the end of the product’s life. This approach allows pharma companies to benefit from the lowest production costs and fewer process issues right from the start. Gaining maximal benefits requires close collaboration between the sponsor and the CDMO LCM teams.”
Salo explains that lifecycle management is part of the business strategy and that efforts and resources should be dedicated to an LCM strategy for the long term. The main element in strategy building is identifying external opportunities and threats. “Internal capabilities, projects and partnerships need to be planned so that opportunities can be converted into business, and risks related to threats can be mitigated,” he says.
One way the experts advise mitigating risk is to assemble a dedicated committee to the LCM strategy. According to Cutting Edge Information, 86 percent of life sciences companies surveyed in 2014 have dedicated teams to manage the lifecycles of their pharmaceutical brands. These cross-functional teams should include representatives from marketing, R&D, manufacturing, process engineering and regulatory affairs. According to Natalie DeMasi, research analyst, Cutting Edge Information, it is important to dedicate teams to brand lifecycle management. LCM strategies are time sensitive, she explains, and delays can derail projects and cost hundreds of millions — if not billions — in lost revenues.
Assembling such a team early in the development process can yield accelerated initial drug development and help engineer an optimal manufacturing process from the start, explains Salo. “Continuous process improvements can then be made to reduce the cost of goods, novel delivery systems and packaging solutions can be identified, and more value is created for patients and payers with improved efficacy, reduced side effects and ease of dosing.”
In one case, Fermion developed an API process for a mid-sized pharma company that had an innovative small molecule pipeline. Fermion manufactured clinical trial API batches, and manufactured commercial API after the product was launched. Two years later, the client wanted to initiate a program to decrease the manufacturing costs of the product.
“They wanted to maximize profits during the market exclusivity period, but also ensure they optimized everything to do with API manufacturing before the product went off patent,” explains Salo.
The API manufacturing process optimization activities included increased batch sizes and yields, removal and shortening of certain unit operations, outsourcing of an intermediate, and optimization of campaigns to reduce bottlenecks. “The project was completed in several steps with careful consideration, not rushing,” says Salo. “In fact, the project took almost eight years, but the cost of goods was decreased by 60 percent. Note, that if one decides to aim for a large, but expedient, decrease in cost of goods, then implementing a large number of resources at once can help reaching that goal.”
Salo adds that Fermion arranges with clients in writing that certain optimization activities will be done only after launch to save time before launch. “Many of our clients have great ideas in improving the process, but their priority is to get the product to the market quickly. We combine their ideas with our knowledge based on experience with similar products and what works well with our facilities, and the result is a diverse lifecycle management program that can be implemented both pre-and post-launch.”
LCM OPPORTUNITIES AFTER PRODUCT LAUNCH
In order to quickly get a medication to patients in need, speed is of paramount importance in development. But opportunities remain to further optimize the value of a drug after its commercial launch, says David Xu, executive director, head of new product planning, Purdue Pharma L.P. “Pharmaceutical product lifecycle management is about maximizing the value of our products to our customers. This includes new indications, improved formulation, new delivery system or packaging, IT solutions, etc., to expand the utility, and improve upon efficacy, safety and the patient experience.”
Xu explains that Purdue’s approach to LCM stems from understanding customers’ unmet needs — including unmet needs from patients, providers, payers, healthcare delivery systems and society. “This understanding improves over time, and the opportunities are ranked for each brand and across the portfolio, in terms of importance and potential impact by cross-functional teams. The selected LCM projects are executed following the same process as a new product in development. Throughout the process, we continue to validate the customer needs and assess the potential solutions against changing market conditions.”
One of those is the opioid market. The Centers for Disease Control and Prevention (CDC) states that opioids are responsible for more than six out of 10 drug overdose deaths, and 91 Americans die daily from an opioid overdose. Xu says Purdue Pharma recognized the significant societal unmet need in addressing opioid diversion, misuse and abuse. An LCM strategy was put in place to reformulate OxyContin, an opioid pain medication for which Purdue held the original formula patent until 2013. “After more than 10 years of exploration and trials, we received FDA approval for the formulation of OxyContin with abuse-deterrent properties.”
ADVICE FROM THE EXPERTS
Salo and Xu recommend the following advice for developing and executing an LCM strategy:
• Begin planning and implementing a lifecycle management strategy in the discovery phase of your product.
• Make sure the LCM strategy is based on creating the most value for the entire network of the pharma industry stakeholders. Different functions may bring different perspectives of the unmet needs and potential solutions. This approach will, in the end, create most financial value to the product owner.
•If using an experienced CDMO, utilize the full expertise of your cross-functional in-house team and enable their collaboration with the CDMO.
• The LCM plan has to be based on true unmet needs. This requires the organization to continuously deepen its understanding of the market and key customers.
• Starting an LCM strategy before product launch enables new indications to be evaluated and prioritized during the product development phase.
“Lifecycle management serves as a vehicle to grow the business, not just to extend the product life,” says Salo.