Save Money, Make Money: IT Integration and Data Exchange
At the end of the day, all pharmaceutical manufacturers are looking to make product for less money. By integrating systems like enterprise resource planning (ERP), material requirements planning (MRP), supply chain management (SCM), quality management system (QMS), learning management system (LMS), laboratory information management system (LIMS) and the like, a company can reduce errors and human touch all the way through a product’s lifecycle. In addition, people resources can then be used for more crucial roles. All of this decreases costs and propels time-to-market.
Absolutely every time I meet with a pharmaceutical manufacturing customer or prospective customer they always bring up IT integration and data exchange: how do I stop the re-work? How do I get my data from here to there? I’m tired of printing it out here, walking it over there, and rewriting it, entering part of it into this other system.
SAVING MONEY, TIME AND REPUTATION
In a nut shell, the biggest concern manufacturers have with data exchange is that they’re tired of re-keying data, and tired of getting things wrong. Manufacturers know that when people re-key data in different non-connected silos (data from systems like ERP, MRP, purchasing, QMS, LMS, LIMs), this can provide a source of error. Transposing data over and over again and redundant data costs money and costs manufacturers efficiencies in their processes.
Systems like ERP and MRP contain part numbers and supplier information; having to re-key that data any place is kind of silly. It is important to get away from this and create the ability to pick a single source of that data and move it around into different systems.
Here is an example of successfully integrated data and systems; there was no need to re-enter data at any step of the process: it is discovered in a MRP system that a source material is not proper and a non-compliant process is kicked off. The batch number is confirmed, and pushed across into the quality management system. Then the quality management system manages the whole process — what needs to be done with this material; is it scrapped? Is it reworked, or is it taken out of production? Once an action is chosen, then an update is triggered back in the MRP system.
PUT PEOPLE WHERE THEY ARE MOST NEEDED
Personnel costs are budget-killers. By integrating IT systems, a company can conserve the cost of human capital: decrease human touch where it’s not needed and put people costs where they are needed — where it does the most benefit. If a company integrates IT systems a person is not needed to do certain, more simplistic jobs, like data entry. Instead, that person can function in areas that are more critical, like talking with customers or suppliers; determining problems; or following the logic through a corrective or preventative action. In other words, a manufacturer shouldn’t have to pay people to do jobs that machines can do; instead put talented people where they are called upon for their judgment, which is what machines can’t provide.
And, with today’s business processes, a person doesn’t even have to kick off a process. A process is started automatically, sometimes even based on input from another system when an exception is found based on business process rules. This means people don’t have to execute some tasks, such as running reports to find errors. Instead, people can spend their time figuring out the causes and remedies.
GOOD PROCESSES ARE REQUIRED
With integrated software systems, good people processes must be established, so inaccurate data doesn’t cascade through everything else. In other words, it is important to get the data entered right in the master (official) sources before it gets pushed out through all other systems. The good news is that this expense or commitment to have good processes around data entry to the master sources is much more cost effective than having same data entered five to six times. And there is less error.
In addition, it is important to note that once a process is validated, it is most likely error free.
HOW TO MAKE IT HAPPEN
Most manufacturers address IT needs by what solves the biggest pains, e.g., a recall, burgeoning supply costs or frequent spec changes. Once a company identifies these pains, there will undoubtedly be opportunities to look at how integrating IT and better managing data can solve these pains. At this point, it is key to identify where the master or official sources of business data are located and make sure to link them. As covered already, this will make sure everything sings from the same sheet of music and information is updated as quickly as possible so there isn’t anything out of sync at any time.
DETERMINE THE MASTER SOURCE FOR DATA
Again, it is important that systems are hooked into a master record, or an authoritative source for each type of data. For instance, one system might have all the customer information (contacts, purchase history), while another has all the supplier contact info and history. This data can then flow into the QMS. So when a complaint about a specific product subassembly is received, the QMS can access data from the other systems (e.g., who received the defective product and who provided the bad component to the manufacturer.) This way, any time a process is started in any other system the process always pulls from the updated, correct information — meaning outdated, incorrect information is never used.
This eliminates one of the biggest challenges to reporting or processes — not knowing where data comes from (was it looked up from the MRP system, from ERP, purchasing?)
And the time savings…if data has to be re-entered throughout a company to different systems, or pieces of paper have to be carried around, it takes forever to run a report. With the right piece of middleware, and having everything connected, running a report could happen in just a few moments. For example, when someone kicks off a process, every system gets updated simultaneously, so no lag. No more mistakenly ordering bad material from a supplier that may not be producing the quality material needed.
MasterControl has been recently integrating some of its own internal systems. The company realized it had the same data stored in multiple systems, and when one system was updated, the others weren’t. Two different sources of information would surface and conflict, or one source would have certain data and another source would not. It was hard to determine the real source of the data. Once MasterControl’s systems are integrated, data is promoted throughout the systems. Quality systems that contain SOPs have linked copies of these SOPs wherever needed in other systems, so when a user opens a SOP they get the most recent version.
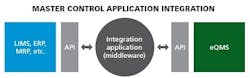
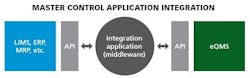
APIs
Some of the most promising innovations going on right now in IT integration include connecting with application programming interfaces (APIs). APIs or database look-ups determine how specific software components should interact and connect (how data is linked) with a piece of middleware. It is easy to build affordable middleware on an API, connecting the middleware into each individual piece of business systems software. Middleware, basically pieces of code that integrate with an API, can be very robust, can update multi sources, can pull from multiple sources, and can really make sure the user gets the exact information they want.
APIs are popular choices because they can be used across firewalls and they usually have access controls (permissions) via connection and login information to protect the source (read-only controls).
Once a manufacturer has defined business processes, integrating IT can be straightforward, considering now all the automation, APIs, different connections, and what is referred to as “the internet of things” (IoT). It is insane to think about how manufacturers used to have to run cable from different machines to try and centralize data. Now everything’s connected — why not use this connection to make sure data is moved around to get it at the right place at the right time?
QUALITY MANAGEMENT SOFTWARE (QMS)
It is worthwhile to highlight specifically integrating QMS with other systems. QMS is the keeper of the quality processes, processes such as validation, deviations, non-compliant, change control, audit and CAPA, which can discover and thwart costly problems. QMS contains all the work done around the quality processes and what was done to prevent issues from happening again.
By integrating QMS with other software systems (ERP, MRP, SCM, LMS, LIMS) and starting quality processes earlier, it leads to finding problems sooner. This leads to increasing quality, obtaining compliance faster, and decreasing the costly things that happen after the fact. In other words, integrating QMS with other business software systems can accelerate overall business.
As a working example, if QMS, LMS and MRP are connected, a quality process can kick off a learning/training exercise, like making sure staff learns a new process or specification. This assures the line gets the correct specs at the correct time to start the new batch. And then the MRP contains the updated/revised material list for that batch or lot number of the materials needed on the line.
Consider this scenario, when lines switch at lunch, making a different product for a different company. The document system connected with the LMS and QMS systems tell a line worker whether or not they have been trained. If the line worker has not been trained, a job aide with the specs for the job will not come up and the operator must call a supervisor to be “‘on the job” trained, and a process started in the LMS to provide additional training for that worker. In another example, the MRP tracking a batch number and specifications for raw materials could trigger an “out-of-spec” process in the QMS system. Once the deviation is discovered, decisions can be made and processes triggered in the MRP system to scrap, rework or adapt the specs to use the material.
THE FUTURE
The pharmaceutical manufacturing industry is learning how to better integrate their software systems. A change happening in the industry is that it is moving out of using Simple Object Access Protocols (SOAP) APIs and compiled apps for middleware and it is moving into using web-based applications that use Representational State Transfer (REST) interfaces.
The pharma manufacturing industry is preparing for the next frontier — automating the entire batch record. Soon every step in the product lifecycle, from sifting the powders, mixing the powders together, compressing tablets, filling the bottles, sealing the bottles and the entire run will be electronically recorded in a single system. This process will collect feedback from equipment along the way and from different sources of information, like the lot number of an active pharmaceutical ingredient, the supplier of that lot number, who made the foil liners, the cotton balls in the bottle, etc.
Instead of hand sign-offs/check-offs, imagine everything will be web interfaced on a tablet — soon there won’t be the need for three different colored pens!
There is a lot to be said for the ambitious school of thought “do it now; don’t wait until you have a problem.” There are major benefits to be had by initiating very simple integrations OR complicated ones. Enhanced IT integration and data management can be handled by internal IT or by an outside service. What is important is that pharmaceutical manufacturers look to these valuable opportunities to grow their business.