BioPharma Manufacturing in the EMEA Region
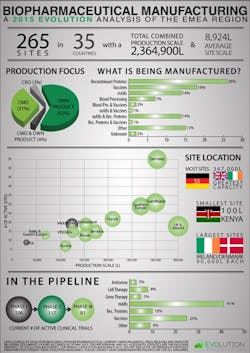
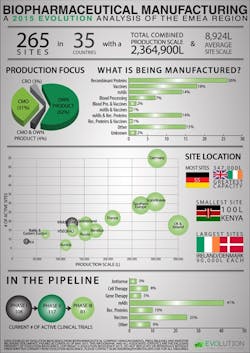
Biopharmaceutical manufacturing facilities are overwhelmingly located in Europe, with only 17 sites in the Middle East and 9 in Africa. Germany is the country with the highest number of sites, with a total of 55 facilities, but their overall production scale is less than that of the combined total of the 18 sites in Ireland and the UK.
The study reveals that European Union companies are producing biopharmaceuticals and biologics at commercial scale with some operating at tremendous capacity. With a combined production scale of some 2.3 million liters, EMEA producers have developed a dynamic presence in the market, something that is also driving demand for highly qualified, experienced and effective professionals to fill critical development and manufacturing roles.