We’ve seen this movie before, and it doesn’t end well.
U.S. pharma companies are finding themselves in a place eerily reminiscent of five years ago. Back then, all of us were fairly certain that U.S. Food and Drug Administration would mandate unit-level serialization at some point…we just didn’t know exactly what the directive was, or precisely when it would go into effect. Although it was evident track and trace was coming, the pharma sector had seen enough false starts (remember “California 2015”?) that initial efforts to comply with Phase 1 of 2013’s Drug Supply Chain Serialization Act (DSCSA) were tepid, to put it mildly. For a long while, pharma companies didn’t really buy in and, because of that, didn’t really buy infrastructure.
It was a recipe for disaster: a series of delays, directives and clarifications to a law requiring large-scale equipment investment, personnel training and production line disruption. Amid the confusion, many pharma companies refrained from commencing the long, expensive trek to serialization until they were completely sure what would be required of them, and when. In hindsight it’s hard to blame them for needing the inevitable enforcement extension, delaying potential penalization until November of 2018.
That said, with mandated aggregation by 2023 a likelihood, can we all agree not to do this again?
Where we are today
Here’s where aggregation stands: the FDA is currently gathering insight from suppliers to precisely define how DSCSA’s next phase, called “Enhanced Drug Distribution Security” (EDDS), should work. For the EDDS phase, the goal is to require all stakeholders in the pharmaceutical supply chain to include unit-level serial numbers with accessible transaction information (TI) on all prescription drugs. A nod to greater transparency and accountability, entities selling prescription drugs will be required to know precisely when unit-level serial numbers are included in their shipments. This will necessitate that the entities have their own serial number-based packaging hierarchy — in other words, aggregated data — which would not need to be provided to customers but would need to be given to any third-party logistics providers.
The DSCSA’s EDDS phase is scheduled to begin in November 2023. And that brings us back to 2020, circa 2015: a crucial yet still-nebulous law with enough time remaining until compliance to trick all of us into thinking we have more time than we actually do.
Though X factors still remain, sufficient direction has been given so that pharma companies can avoid being part of the inevitable last-second dash to aggregate. If 2018 was a horror show, why star in the sequel? Here are just a few reasons why pharma companies should aggregate now rather than panic later.
Aggregation is more complicated than unit-level serialization
Explaining why implementing aggregation is more complicated than serialization is, ironically, quite simple: Serialization is vertical whereas aggregation is horizontal. The former is an effort largely undertaken under one’s own roof; the latter is an inter-company operation forged in conjunction with limitless partners, companies and oversight entities. As a discipline, aggregation will tighten operational practices and allow pharma companies to strengthen quality assurance checkpoints.
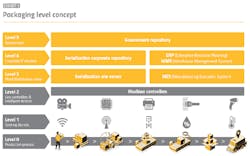
Serialization can be described as a “levels ladder,” with the first three steps comprising the infrastructure to serialize (vision systems, printers, etc.) and the organizational setup necessary to sync up company-wide traceability initiatives. Level 4 serialization was the “enterprise” step in which this data could be properly reported.
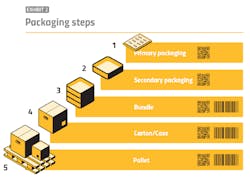
By contrast, aggregation is a far more communal process in which entities must, from an information integration standpoint, stand on each other’s shoulders. Each participant in the supply chain receives necessary data from a predecessor, then supplements this with its own data. This data includes aggregation data of the primary, secondary and tertiary packaging. Aggregation is a maze that can potentially involve not only the original packager and various re-packers, but also reimportation entities and parallel imports.
Back at the manufacturing facility, aggregation also presents challenges to packaging line speeds, since it involves the labeling and tracking of packaging hierarchies. Discerning among various manual, automatic and semi-automatic aggregation methods will invariably require time and expertise to define line throughput obstacles and, from there, mitigate them as best as possible to maximize overall equipment effectiveness (OEE). Here, the wide-ranging factors — as well as their relatively recent appearance on the production landscape — place vendors with sufficient aggregation experience at a premium.
By not aggregating now, you’re limiting customer and partner pools
There are far more reasons to aggregate than “because the FDA said so.” Aggregation does the following:
- Enables manufacturers to cater to multiple countries’ track and trace requirements
- Empowers supply chain stakeholders to significantly improve warehousing and inventory management
- Allows for simplified shipping, commissioning and decommissioning of cases and pallets
- Provides complete visibility into product recalls and returns
- Increases transparency throughout the supply chain
- Allows geo-fencing/geo-tracing of product within the supply chain
Many U.S. distributors have recognized this and redefined their rules of engagement accordingly, preferring to do business only with pharma companies who have, at least to some degree, adopted aggregation elements. And as the number of pharma distributors requiring partners to be aggregation-ready continues to grow, the further behind pharma companies who haven’t begun the process will be — because getting up and running takes time, training and an extensive trial-and-error approach.
Implementing aggregation requires significant end-of-line alterations, introducing new machines and checkpoints, creating associated processes and educating operators on new procedure. Often, aggregation will entail additional operator intervention, which increases process time and batch rejections and, therefore, affects overall line output. Aggregation also restricts multiple existing packaging processes, as well as certain reconciliation methods. Finally, SOPs and solutions to exceptional situations must be realized and settled upon.
In short, aggregation is increasingly becoming an exclusive club, and gaining membership requires significant time, training and money.
Data: The good, the bad and the necessary
Like serialization, implementing aggregation will produce troves of new data. Some of it is obligatory, some of it seems like spillover. But all of it can be advantageous if properly translated into actionable intelligence. The potential benefits have coined a new industry term: “value beyond compliance,” alluding to means of leveraging mined data for operational efficiency, marketing, consumer touchpoints, etc.
At its core, the point of aggregation is inter-partner transparency. Aggregation allows various invested entities along the supply chain to verify that collaborators are on the level and on the ball. The results are living, breathing accountability documents — digital databases of who did what and when to products with common stakeholders.
But the data produced for aggregation compliance also has value at its source: the manufacturing plant.
First and foremost, the data produced by aggregation can be used to reclaim some of the OEE lost to aggregation. Aggregation is, like any new element added to a packaging line, likely to cause some slowdown. However, by properly capturing and analyzing the data that this new system provides, some speed can be recovered by understanding where the slowdowns are occurring and, to the extent possible, rectifying mini situations that can add up to significant sticking points.
It’s simple: The same tools required for pharma companies to “show their work” to partners can be utilized to reveal potential for increases in efficiency within the production plant itself. This comprehensive approach to data is at the core of Pharma 4.0 “factory of the future” technology. It’s where the industry is going — why not get there sooner rather than later?
The best vendors will have longer lead times as 2023 approaches
The last thing pharma companies want to do is…well, be last.
As the November 2017 date for unit-level serialization compliance neared, pharma companies found themselves in a worst-case game of hurry-up-and-wait: a looming, mission-critical mandate with lead times from industry-leading vendors that far exceeded the deadline. In fact, even the extra year’s extension didn’t clear the capacity logjam that understandably transpired. For an effort of this magnitude and expense, pharma companies wanted to partner with trusted vendors; in fact, many of those that didn’t, for lack of time or resources, wound up with inferior products, service or even suppliers that went out of business shortly thereafter.
The runup to 2023 will be no different. Pharma companies happy with their serialization systems have no reason to delay reengagement for aggregation. Companies unhappy with their vendors have even less reason to delay: Now’s the time to find a vendor who is up to the task.
Implementing something as comprehensive as aggregation requires experience on several levels. First and most obvious, a vendor should have exemplary aggregation hardware and software products, and have an existing customer base that can attest to that quality.
Next, since aggregation impacts the entirety of a packaging line, vendors with experience in overarching line integration have a leg up. Among other benefits, these partners have experience training personnel to significantly minimize per-item aggregation cycle times.
Last but not least, remember that the U.S. isn’t the first to the aggregation dance. Even if they aren’t the biggest names in America’s track and trace market, vendors originating overseas but with significant presence in the U.S. are worth considering. Companies that are headquartered in a nation that already has successful aggregation requirements and that cater to global pharmaceuticals exporters have experience outfitting packaging lines with aggregation that complies with country-specific guidelines from around the world. That sort of flexibility and familiarity are big benefits for U.S. pharma companies with international aspirations.