Cold chain logistics plays a vital role in the pharmaceutical industry. One of the most notable ways is in the transportation and storage of temperature-sensitive products like vaccines, blood products and biologics. Without a doubt, the pandemic brought challenges to the pharmaceutical cold chain — many for which the industry was not prepared.
The urgent need to distribute vaccines and other perishable supplies highlighted the vulnerabilities of the existing supply chain. The regulations governing cold chain logistics have undergone major shifts in recent years, aiming to maintain the quality and safety of the critical raw materials essential for delivering lifesaving therapies. Shipping these materials in certain locations or by certain modes — particularly air transport — increases vulnerabilities due to air pressure and temperature fluctuations. Improper packaging could lead to heat or cold exposure outside of acceptable temperature ranges and potentially compromise the quality of the product.
To safeguard the integrity of pharmaceutical products throughout the supply chain, organizations are adopting Good Distribution Practices (GDP) and Hazard Analysis and Critical Control Points (HACCP) principles. GDP guidelines outline the necessary processes, infrastructure and controls needed. HACCP, on the other hand, focuses on identifying and managing potential risks and hazards in the cold chain process.
In addition to the adoption of GDP and HACCP guidelines, advancements in technology and monitoring and the development of smarter preservation methods have played pivotal roles in safeguarding temperature-sensitive pharmaceutical products.
Finding the right preservation methods
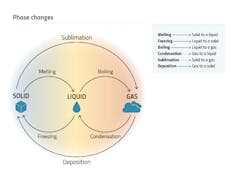
Preserving goods throughout the cold chain has been an ongoing challenge. However, the industry has been actively exploring smarter preservation methods to ensure product quality. One notable development is the increasing use of dry ice for shipping temperature-sensitive products. Dry ice, which is solid carbon dioxide, has excellent cooling properties and is commonly used as a coolant during transportation. It sublimates directly from a solid to a gas, eliminating the need for melting ice or refrigeration units. This feature makes it a smart choice for maintaining the required temperature conditions during transit. But shipping cold chain products using dry ice comes with certain limitations. For instance, traditional methods of using dry ice may result in inconsistent insulation and temperature control, potentially jeopardizing product integrity. To overcome this challenge, some companies are investing in packaging solutions that provide enhanced insulation, better temperature control and longer-lasting cold conditions. These advancements minimize temperature variations and ensure the safe delivery of temperature-sensitive pharmaceutical products.
Evolution of technology
The evolution of technology for shipping cold chain products has produced significant advancements in recent years. Manufacturers and logistics providers have continuously strived to enhance insulation, temperature control and overall product integrity. One of the key areas of improvement has been the development of more efficient packaging solutions. Insulated shipping containers with enhanced thermal properties help minimize heat transfer and temperature fluctuations during transportation. These containers are designed to withstand varying ambient conditions and can maintain the required temperature range for extended periods. Furthermore, innovative temperature-monitoring devices have been integrated into the packaging, allowing real-time monitoring and data logging of temperature conditions.
This enables stakeholders to track and analyze temperature data throughout the journey, ensuring compliance with regulatory standards and identifying any potential temperature excursions. To further address the limitations of traditional dry ice packaging, companies have also introduced advanced phase-change materials (PCMs) and thermal wraps. These technologies enhance insulation and provide longer-lasting temperature control, thereby extending the shelf life of temperature-sensitive products. A PCM is a substance that absorbs energy at the phase transition to provide cooling. A simple example of using PCM would be ice in a drink. Two of the most used PCMs in transportation are water-based gel packs and dry ice (solid carbon dioxide). Dry ice is regulated as a hazardous material in transport because the sublimating carbon dioxide gas displaces oxygen. Advanced PCM gels are used to replace dry ice and have a phase change around -1 degree C or -20 degrees C. Replacing a hazardous material in transport with a non-regulated gel provides some transport restriction relief. Thermal wraps are enhanced insulation that provide longer-lasting temperature control, protecting temperature-sensitive products from extreme temperature shifts during transport. These can be added to the existing thermal protection during extreme situations.
A global pandemic meant global solutions
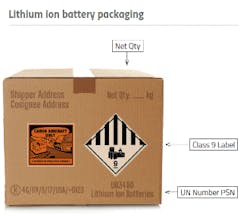
The global supply chain disruptions, transportation restrictions and increased demand experienced during the pandemic put immense pressure on pharma companies and logistics providers to find alternative solutions to deliver critical products to health care facilities and patients in a timely manner. Companies and industry stakeholders rapidly adapted to the challenges by implementing agile strategies and leveraging technology to ensure uninterrupted cold chain operations. This included optimizing transportation routes, enhancing inventory management systems and establishing dedicated cold storage facilities. Moreover, collaborations between pharma companies, logistics providers and government agencies played a crucial role in streamlining the distribution process, prioritizing high-demand regions, and reducing bottlenecks. Examples include the transport industry and regulators quickly adjusting to provide solutions and regulatory relief for new companies producing and transporting hand sanitizer (hazard class 3). Amazing collaboration also occurred between air carriers, regulators and pharma logistic providers to move the large quantities of dry ice (hazard class 9) needed to maintain frozen vaccines during transport. There are several dangerous goods (DG) transport associations, such as the Council on Safe Transportation of Hazardous Articles (COSTHA), Dangerous Goods Advisory Council (DGAC) and Dangerous Goods Trainers Association (DGTA) working to streamline transport requirements and prevent regulations from impacting pharma companies. One example would be recent work by several organizations at the UN to simplify the requirements for lifesaving medical devices containing lithium batteries in transport.
Steps to ensure compliance
Another challenge when it comes to the pharmaceutical supply chain is that many of the items being shipped — from clinical specimens and medical devices to dry ice and temperature monitors containing lithium batteries — can be classified as DG and have strict regulations around how they should be packaged, handled and transported. To ensure successful shipping and proper handling of goods across the cold chain, it is important to keep the following strategies in mind:
1. Know the regulations
Stay updated on the specific items being shipped and their classification, especially if they are considered dangerous goods. Familiarize yourself with the regulations governing the transportation of these items, including any shipping restrictions. It’s also important to establish processes and infrastructure to ensure compliance throughout the supply chain. This includes staying informed about evolving DG shipping regulations in the U.S. and globally, and effectively communicating relevant information within your organization and with supply chain partners. A notable example is the change in regulations regarding the transport of lithium batteries as a hazardous material. The lithium battery found in a medical device, temperature recorder or laptop is regulated in transport. New hazard communication and modal restrictions could stop shipments (affecting consumers) and subject companies to significant fines and penalties for noncompliance. The Pipeline and Hazardous Materials Safety Administration (PHMSA) offers a lithium battery guidance document to guide U.S. companies in safe and compliant lithium battery transport.
2. Seek expert guidance
DG shipping regulations can be intricate and change constantly. Consult various attorneys and compliance experts to ensure compliance with the regulatory requirements for shipping pharmaceutical products across different modes of transport. These experts can help assess your facilities and compliance programs, identify any gaps, and integrate compliance into aspects of your organization and supply chain partnerships.
3. Use appropriate packaging
Proper packaging is crucial to safeguard the integrity of goods during transportation. Utilize only materials that are UN-certified for DG packaging. Supply chain issues have been devastating to many suppliers of UN-certified packaging since substitutions are often not allowed in certified packaging. One hotly discussed topic recently in life science packaging is the UN requirement for fiberboard boxes used in limited quantity packages to be able to pass the Cobb water absorption test. This test is difficult for some recycled fiberboards to pass, which comes at odds with the sustainability requirements to use recycled materials that many companies are implementing.
4. Provide effective training
Training is vital in ensuring safe and compliant shipping practices. Ensure that all employees involved in shipping and handling DGs receive the necessary training, not only to fulfill training mandates but also to equip them with the skills to perform their duties correctly. Incorporate digital and e-learning tools as well — these can facilitate remote training for new employees and offer interactive and effective learning experiences.
5. Foster a compliance oriented culture
Pharmaceutical companies have a direct impact on people’s lives. It is crucial to maintain accountability and uphold the highest standards. Any indication of cutting corners, noncompliance, or inadequate processes can negatively affect customers or patients, brand reputation and business outcomes. Therefore, strive to create a culture that prioritizes compliance and embraces the necessary measures for ensuring safe and compliant shipping practices.
A promising future
Although the pandemic posed challenges, it has also spurred innovation and resilience within the supply chain, driving the evolution of technology in shipping cold chain products. As industry continues to adapt and improve, the future of cold chain logistics holds the promise of even greater efficiency, reliability and safety for the delivery of critical medical products.
About the Author
Jay Johnson
Senior Manager of Labelmaster Services, Labelmaster
Jay Johnson is senior manager of Labelmaster Services at Labelmaster, the leading provider of labels, packaging and technology for the safe and compliant transport of dangerous goods (DG). As an expert in DG regulations, Jay specializes in packaging and logistics solutions for the chemical and biotech industries. Jay has been an active participant and major resource in the development and implementation of international DG regulations. He is a member of the Council on Safe Transportation of Hazardous Articles (COSTHA), a board member of the Dangerous Goods Trainers Association, Inc. (DGTA) and past chairman of the Dangerous Goods Advisory Council (DGAC). He is also a Dangerous Good Safety Advisor (DGSA) in Europe and an approved dangerous goods instructor by the UK Civil Aviation Authority.