The last day of an industry conference isn’t known for being crowded. But as Friday stretched on inside one of the large meeting rooms at the Bioprocessing Summit in Boston this August, it remained difficult to find a seat. The focus of the day’s full schedule of speaker sessions? The vexing issues behind scaling up production of gene therapies.
It’s no surprise that interest in this topic is high. Although it’s only been two years since the U.S. Food and Drug Administration made its first approval of a gene therapy, the market for these often one-and-done treatments has since expanded rapidly. In 2017, there were an estimated 391 gene therapy companies in the U.S. This year, nearly 100 more have jumped into the game and every major player in Big Pharma is now clawing for a piece of the pie.
On the surface, opportunities to cash in on the explosive growth are popping up all throughout the supply chain — from new facility design to contract manufacturing. But behind the scenes there’s a bit of anxiety about whether or not the industry can keep up with its own efforts to commercialize.
“If we’re talking about rare diseases, then manufacturing will be straightforward,” Luis Maranga, chief tech operations officer at Voyager Therapeutics told the audience during his presentation at the Bioprocessing Summit. “But if you think about a disease like Alzheimer’s — there are currently about 5 million patients, which will rise because the population is aging. Then, if we are trying to treat thousands of patients, that’s when it’s hard. As a field, we have to start preparing for it today or we’re going to be in a worse situation.”
As this tidal wave of new treatments moves closer to making landfall, the industry is still working furiously to overcome the numerous challenges of commercialization such as securing raw materials, finding the right equipment, recruiting expertise and all the other issues associated with scaling up production from the lab into the pharma plant.
Patients are waiting. The FDA has been providing guidance to lead gene therapies down a path to approval. But as more gene therapies launch onto the scene, what will it take for the industry to stick the landing?
Building the gene machine
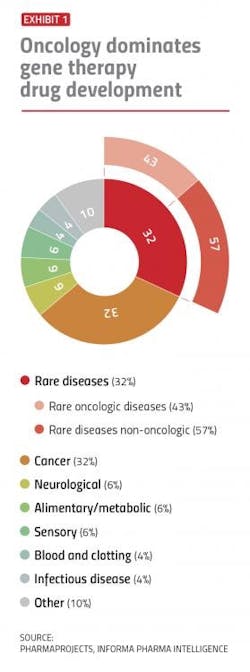
There’s no question today that gene therapies are one of the keys to treating and potentially curing inherited diseases — from genetic disorders to cancer. But it’s been a rocky road getting gene therapies to go mainstream.
Scientists have been tinkering with ways to cure diseases by targeting genes for the last four decades. On the heels of the development of recombinant DNA technology — DNA grown in a lab using genetic material from multiple sources — a seminal research paper emerged in 1972 which laid out a groundbreaking idea for treating genetic disorders by introducing functional DNA into a patient.
It took 20 years of additional research before the idea became a reality in 1990 with the approval of the first gene therapy study in the U.S. The patient was a 4-year-old girl with a rare genetic disease that left her without a key enzyme for preventing infections. As with most gene therapies in development now, the patient was administered replacements for her faulty gene using viral vectors. Because viruses are so adept at invading the body, viral vectors continue to be the most commonly used way to deliver gene therapies into a patient’s cells.
Ultimately, the first gene therapy trial was a success and the young patient was able to live a normal life outside of isolation. But the field suffered a major setback eight years later when an 18-year-old patient in a gene therapy study died after suffering a severe immune reaction to his treatment.
Despite the shock and public concern following the patient’s death, the gene therapy market staged a careful comeback. By 2003, the first gene therapy was approved for head and neck cancer in China. Ten years later, Europe became a hotbed of gene therapy R&D and now, the boom has officially hit the U.S.
Today, gene therapies encompass a range of treatments often referred to as “advanced therapy medicinal products” including genetic editing techniques and CAR T-cell therapies, which are gene and immuno-therapies, and work by using engineered cells to boost a patient’s own immune system to fight diseases like cancer.
A research scientist transferring contents from roller bottles to vials in the Spark Therapeutics laboratory. Image: Spark Therapeutics
In 2017, the FDA approved the first CAR T-cell therapies in the U.S.— Novartis’ Kymriah and Kite/Gilead’s Yescarta, which both treat cancer. Then in December of that year, Spark Therapeutics, a biotech upstart based in Philadelphia, became the first company to ever have a gene therapy for a genetic disease approved by the FDA.
Designed to treat a rare kind of retinal dystrophy, Spark’s Luxturna is a one-time injection that works by replacing a mutated RPE65 gene that can cause complete vision loss over time. In one study, 90 percent of the patients treated with Luxturna experienced improvement in their functional vision one year after treatment.
So, what’s it like when you spend years developing a game-changing drug like Luxturna and then finally find out if your company has won an FDA approval that could change the course of pharma history?
“You get a fax,” Diane Blumenthal, head of technical operations for Spark, explains with a chuckle. “It was right before Christmas and Spark’s head of the regulatory board called me to tell me it was approved, but it was a bit anticlimactic because there were only a few people in the office.”
But within weeks of Luxturna’s approval, the excitement over the first FDA nod for a gene therapy in the U.S. was quelled somewhat by news of the treatment’s price: $425,000 per eye.
The price isn’t right
The approval of Luxturna not only set up Spark to become one of the first companies to dive deep into the many challenges of commercialization in the U.S. — it also became the first that had to justify its price tag for a therapy.
Drug companies have long stood behind the argument that the price of innovative treatments are set to recoup the high, often decades-long costs of innovation — while funding future research, as well. Some argue that potential one-time cures like Luxturna could also end up being cheaper in the long-run than a lifetime of paying for other treatments. And of course, innovative biotech start-ups like Spark also tackle the time-consuming and costly research for rare disease treatments that Big Pharma often avoids – if they’re not going to do it, who will?
But now, when a gene therapy comes onto the market, it becomes the world’s new “most expensive drug” (the crown was most recently passed to Novartis’ Zolgensma which treats an inherited form of spinal muscular atrophy for about $2.1 million).
So far, gene therapy companies are coming up with new kinds of payer plans so that patients are not denied treatments — such as annual payments, and/or payments tied to the long-term efficacy of the drug.
“Just because you can give a patient the treatment, doesn’t mean that they will have the desired outcome,” says Patti Seymour, managing director of Industry Specialty Services practice, biotechnology consulting group at BDO, a global accounting and consulting firm. “They need to show that the gene therapy has been stably incorporated into the patient’s genome and it has a durable response. What if they need a second dose? Do you charge a sliding scale? [This kind of outcome-based payment system] is what economists are working on.”
For now, the long-term impact of these “ultraexpensive” treatments on the insurer and health care system are still unclear. But one thing is for sure: Gene therapy companies need to find ways to improve the efficiency of production to help drive down the price of their treatments.
Growing pains
It’s not uncommon for the cost and complexity of manufacturing to be high in a nascent industry launching products made with an innovative technology. For the gene therapy industry, figuring out how to now make large-scale production run as smoothly and cheaply as possible with the market’s existing technology is top of mind.
“Bringing down the cost of these therapies is a big goal for companies right now,” explains Luca Mussati, vice president, Pharma and Biotech at Exyte, a design and engineering company. “For the industry, the goal is to lower manufacturing costs by ten or twenty fold.”
Inside the Bioprocessing Summit, the entire section of the conference focused on gene therapy manufacturing was devoted to viral vectors — one of the hardest aspects of gene therapy development to scale up. For two days, dozens of companies gave presentations on how they were finding ways to get higher yields of viral vectors from their process, with most focusing on the more commonly used adeno-associated virus (AAV). But with many companies going about it in different ways, it was clear that the industry has yet to settle on a standard approach.
Upstream challenges
There are several phases and platforms involved in producing viral vectors. At the heart of the challenge in gene therapy manufacturing is the difference between producing the vectors using adherent cell lines versus suspension.
“Traditionally, viral vectors were expressed in cell lines using adherently grown cells on substrates, which was a very tedious process,” explains Amanda Micklus, an analyst with Informa Pharma Intelligence, a pharmaceutical research and analysis firm.
Adherent cell lines work well in an academic setting, where gene therapies have traditionally been developed, but are typically considered too labor- and equipment-intensive for wide-scale use. Now, the focus is on transitioning the process to growing the cells in suspension with bioreactors.
According to Ryan McDonough, the biotech market sector lead for CRB, an engineering, architecture and construction firm that works on designing and constructing biotech facilities, suspension works better for scaling up because companies don’t necessarily have to increase the square footage of their facility in order to boost their production.
“Suspension cell culture is more typical in the traditional biopharma marketplace. With suspension, scaling up requires larger bioreactors instead of additional bioreactors, which is typical with adherent cell lines,” McDonough explains.
When Spark launched Luxturna, the company used a commonly utilized adherent process that works by attaching the cells to the sides of roller bottles. Blumenthal says the process has worked for Luxturna because it treats a small patient population. Now, the company plans to switch to suspension for the other treatments in its development pipeline — including gene therapies for hemophilia A and several central nervous system disorders.
“Roller bottles have been used for many decades for vaccines,” Blumenthal explains. “But if you can get the process to work in a suspension culture, that’s a much better way to go.”
Another unique aspect of gene therapy manufacturing is the use of transient transfection, which is how DNA is delivered to the cell being used to make the virus. Plasmid DNA is the key raw material used in transient transfection and until the process is optimized to reduce the number of plasmids required, the increase in gene therapy production is putting pressure on plasmid-makers to keep up.
“Everybody is now keeping a close eye on plasmid manufacturing because there are not many companies that have the capability to make cGMP-grade plasmid,” Blumenthal says.
Downstream complexity
Frustratingly, the downstream purification process of separating viral vectors from contaminants can also create yield losses up to 70 percent. Often, the low vector recovery is due to the mixture of full and empty capsids (the protein shell of viruses). There’s also no consensus about what to do with empty or partially full capsids and if they should be treated as contaminants. In general, capsid chemistry is a part of the process that Blumenthal says the industry is still figuring out.
Once the vector production is complete, it also has to be stored and transported in temperatures of -65 degrees Celsius or colder, which adds another layer of complexity.
“Transporting larger quantities of product under these conditions can be challenging,” Blumenthal says.
Overall, the viral vector manufacturing process is in need of a technology upgrade in order to work more efficiently on a bigger stage. In addition to the particulars of making the vectors, McDonough says that companies are also looking for ways to improve process closure.
“The industry needs the ability to manufacture these cell and gene therapies in a manner where the process is never exposed to the environment,” he says.
Several industry experts also say there is a huge push to bring more automation and robotics into vector manufacturing to minimize the need for human intervention and speed the process along.
“There’s also a need for a more robust analytics package to test products and improved formulations to keep them stable for long periods of time,” Seymour says.
So far, the general consensus is that equipment vendors aren’t always keeping up with the pace of commercialization. But the demand for new technologies is there and the race is on.
“We’re expecting to see some truly transformative technologies in this space, because it needs to happen,” Seymour says.
The path to commercialization
One common refrain from companies that have now “been there, done that” in gene therapies is that drug developers should make decisions about how they want to scale up early in the process. It’s a tricky proposition, particularly because it’s difficult to predict how much capacity they’ll need to meet future demand.
“One of the biggest challenges for manufacturers is that they don’t know yet what the rate of production will be, or what technology they’ll need to produce their medicine,” says Stefan Kappeler, technology manager, Life Sciences at Exyte. “Companies want to make sure they have the best process laid out. Often, they just use the technology that’s on the market at the moment. But one year later, there might be a big advancement in that technology that they then need to stay competitive.”
In addition to seeking out the right technologies, drug developers also have a big decision to make when it comes to finding a facility.
“It helps to develop a commercialization strategy that starts with a ‘make versus buy’ analysis — i.e., building a facility for your own use versus buying manufacturing capacity from another company,” Seymour says. “This has to be done early in the process, very often before you have a clinical signal. It’s a big decision with multimillion-dollar consequences.”
Of course, companies can also consider working with an outsourced manufacturing partner, and several CMOs have been working to increase capacity, often through mergers. In fact, rising demand for viral vectors was a catalyst behind two of the industry’s biggest acquisitions this year — Thermo Fisher’s $1.7 billion purchase of Brammer Bio and Catalent’s $1.2 billion deal to buy Paragon Bioservices.
But Seymour cautions that at the moment, CMO capacity for gene therapy manufacturing is stretched thin because demand is high, and it could be several years before contract manufacturers catch up.
When a company decides to construct its own facility, several new options have emerged to help drug developers design a space with the right kind of flexibility for the gene therapy market. And if CRB’s business is any indication, it’s a direction that many companies are choosing to go.
According to McDonough, the design and construction firm has seen a 25 percent uptick in gene therapy inquiries since the first wave of approvals in 2017. McDonough says CRB, which worked on the largest dedicated cell and gene therapy facility in the world, also has several gene-related projects in the pipeline that are over 200,000 square feet.
“We can also do operations improvement to help companies understand how to get more out of their manufacturing process with a minimal amount of facility modifications,” he says.
This fall, Exyte is also launching a brand-new, pre-fabricated gene therapy facility called Rita. The company says that the idea is for Rita to work much like Lego blocks with standard components that are highly customizable and can fit together smoothly in whatever way a company needs.
“It’s a concept made up of cells with standard arrangements that can be assembled in various ways for virtually any layout, whether it’s a segregated or ballroom approach,” Mussati says.
The Rita facilities will also be designed so that the customer’s unique facility features and technologies can easily fit into the spaces within the cell.
“Rita is about creating a platform for utilities and infrastructure that can vary from one customer to the next,” Mussati explains. “Rather than adapting the process to the facility — it’s a facility that adapts to the process.”
Mussati and Kappeler say that Exyte predicts that the demand for Rita — along with an equivalent platform the company is launching for monoclonal antibodies — will be so high that it will comprise 20 percent of the company’s revenue stream for its Life Sciences division next year.
What the future holds
The industry has faced similar challenges before. In fact, several experts pointed out that right now, the gene therapy market looks a lot like the market for monoclonal antibodies after they first started hitting the scene. As drug developers worked to transition their process from the lab to the plant, the challenges of scaling up created several stumbling blocks.
“I would see the gene therapy market as being a bit chaotic for the next few years,” McDonough says.
Among all the experts interviewed for this article, the prediction of when the gene therapy industry will find its groove was the same: about five years.
For now, companies are mobilizing quickly to fill in the gaps for gene therapy commercialization. Mergers and acquisitions have increased throughout the supply chain so that companies can marry their capabilities to develop the right tech and quickly increase capacity.
As the industry moves forward, the overarching goal is to create a repeatable platform for gene therapy manufacturing that many companies can replicate. And equipment manufacturers are also working together at an increased rate to innovate new solutions.
“I think the collaboration has been remarkable, especially for the speed at which the market is growing,” McDonough says.
Many involved in the gene therapy market also say that the FDA has been effective at creating a roadmap for companies looking to commercialize gene therapies.
“I think the FDA is providing appropriate guidance and is a great partner with the industry right now,” Seymour says.
Despite the challenges, the payoff for successfully navigating the gene therapy market and commercializing a new product will likely be big — both for the company and patients.
“Throughout my career, I’ve overseen the approval of six new drugs. It never gets old,” Blumenthal says of making it to the finish line with Luxturna. “But this one was particularly special.”