The FDA’s PAT initiative, in combination with Quality by Design (QbD), encourages the pharmaceutical industry to intensify its focus on efficiency in manufacturing. Conventional production relies heavily on off-line testing of both in-process materials and the end products. The FDA seeks to modernize practice by promoting a shift towards knowledge-based design and development, and the use of analytical tools that can continuously monitor critical processes. For some variables, timely and/or continuous measurements in the operational environment remain a significant technical hurdle, but for others, proven solutions are already commercially available. Particle size is one such variable.
For particulate pharmaceutical systems, particle size is often a critical parameter. It determines drug release characteristics, and can also affect other attributes such as flow, suspension stability and mouth feel. Consequently, particle size is routinely measured in powder-related processes and in many instances laser diffraction is the analytical method of choice. Laser diffraction is a fast, reliable and reproducible technique that is equally suitable for off-, at-, in- or on-line analysis. It can therefore be used from early-stage development, right through into pilot and full-scale production.
The benefits that accrue from using real-time laser diffraction particle size analyzers to monitor milling processes are well-documented and include: increased throughput, better product quality, and reduced wastage. The technology is equally relevant, and beneficial, for other common unit operations such as spray drying. In this study, at- and in-line particle size analyzers were used to monitor and control a pilot-scale spray dryer producing oily microspheres.
Producing Oil-encapsulated Microspheres
Spray drying is widely used throughout the pharmaceutical industry and is a valuable technique for the microencapsulation of a solid or oily liquid. Microencapsulation allows control of the release rate of an active pharmaceutical ingredient and can also prevent degradation and oxidation of the core materials, since the outer coating forms a protective physical barrier. It is an equally useful technique for masking the taste of unpalatable formulations. However, to achieve the required performance characteristics, the particle size of the product microspheres must be tightly controlled.
In this study, the aim was to produce oil-encapsulated microspheres in the size range of 10 to 35 microns. Conventionally, microsphere size is controlled using off-line analytical data produced by periodically sampling from the process. In this case, off-line measurement involved light microscopy interfaced with image analysis. Such a technique is useful for early stage research and development because it provides visual images and valuable information about particle morphology and shape. It is also an effective tool for validating alternative sizing methods.
However, light microscopy and image analysis are not ideal for process monitoring and control. Only a small amount of sample is measured, so data quality is heavily dependent on the extraction and analysis of representative samples. Equally important is that the sampling and measurement process is too slow for real-time monitoring.
Consequently the decision was made to investigate the capabilities of at- and in-line laser diffraction analyzers for improved process monitoring and control.
At- and In-line Particle Sizing Technology for Spray Drying
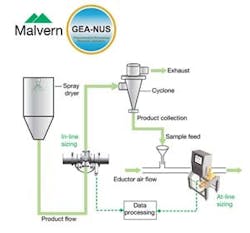
An Insitec system from Malvern Instruments was selected for this application. The Insitec range of laser diffraction particle size analyzers incorporates at-, in- and on-line options specifically designed for the process arena. These systems are suitable for both wet and dry streams with particles in the size range 0.1 to 2000 microns. For this investigation, the same instrument was used, at different times, for both in- and at-line measurement.
Figure 1: Layout for at- and in-line analysis of the exit stream from
the spray dryer.
When installed in the exit line from the spray dryer (Figure 1), the analyzer measures the entire exiting flow, in its “in-process” state, with no sample preparation. For units with larger throughputs, this may not be an option, in which case on-line analysis, where a portion of the process flow is diverted for measurement, is more appropriate. The in-line system is totally enclosed reducing any risks associated with exposure to the process material. Analysis is fully automated so operator input is minimal, and reproducibility is very high.
For at-line analysis, the instrument is installed close to the product outlet conduit. Samples are extracted manually from the process for measurement, as and when required. In terms of process relevance, this approach shares some of the limitations associated with off-line analysis - although response times are very much faster. Also, it is not possible to recover sample after analysis which could be an issue if the material is extremely valuable or in short supply.
In both configurations, an air purge stops particles adhering to the surface of the optical lenses, so maintaining measurement accuracy. With the at-line system, an additional air supply (introduced via the venturi that is used to draw the sample into the analyzer) provides dispersion before measurement. Careful setting of the flow rate ensures break up of any agglomerates without causing attrition of the primary microspheres.
Monitoring the Process
Figure 2: Real-time particle size data measured during start-up of the spray dryer.
Real-time data recorded during start-up of the spray dryer are shown in Figure 2. Periods of transient operation, start-up, shut down or a change of product specification, for example, are almost impossible to track effectively with off-line analysis because of the time lags involved. For this process, start-up was estimated to take 10 minutes, so output during this initial period was discarded in order to preserve product quality. The in-line results show that in fact a steady state is reached much more rapidly than this, in just a couple of minutes. Here then the in-line instrument provides the data required to make a precise decision about when to switch over to product collection, thus cutting waste and maximizing plant output.
Because the in-line analyzer tracks the process in real time, the operator can immediately detect a problem, observe the effect of a planned change, or monitor and correct any long term drift. Examining operation over a slightly wider timescale highlights the ability of the instrument to identify process upsets (see Figure 3).
Figure 3: Identifying a process upset.
Around 10.57 AM, particle size suddenly increases dramatically and there is an associated fall in transmission. This detected ‘event’ correlates with a process action: percussive release of powder from the walls of the measurement vessel. During processing, the oil-coated microspheres, which have very sticky surfaces, adhere to the vessel walls forming agglomerates. To prevent excessive material build up, it is normal practice to tap the vessel with a mallet or air hammer to release the powder. This intervention causes a surge of material through the measurement zone reducing transmission, which is a measure of how easily light penetrates through the sample. Particle size increases because of the nature of the released material (partially agglomerated).
Being able to detect both planned and unplanned process upsets and assess their impact is valuable. For this process, for example, the frequency of any action taken to clear built up material will impact the amount and size of particles released during the procedure and also product homogeneity. In integrated plants, process upsets can also adversely affect downstream unit operations. PAT solutions that offer continuous measurement and high data acquisition speeds capture in fine detail the dynamics of the process, providing information to support a Quality by Design (QbD) approach.
Although the system clearly allows the operator to confirm steady state operation, or otherwise, a comparison of in- and at-line data reveals noticeable discrepancies between the two sets of results. In-line analysis shows a bimodal particle size distribution, while at-line results give a much narrower distribution with almost no second, larger particle size mode. The at-line mean particle size is much smaller than the in-line equivalent. These results are directly attributable to the state in which the sample is measured. In-line the analyzer measures the process with no sample preparation, correctly detecting the presence of agglomerates in the exiting stream. The oily nature of the microspheres, coupled with their small size, makes them prone to agglomeration and as a result, discrete particle size measurement is much more challenging. The bimodal distribution is particularly marked at high oil loadings when this tendency is more pronounced.
The at-line results suggest the air flow used for sample dispersion successfully breaks up the majority of the agglomerates, thereby largely eliminating the second peak of the bimodal distribution. Thus, the at-line results can more accurately reflect the size of the discrete microsphere particles. However, the data from the in-line analyzer more accurately reflect the true nature of the exiting stream. Product from the spray dryer will contain a significant amount of agglomerated material so if the objective is discrete microspheres, then further processing will be needed.
Real-time Measurement of Agglomerating Particles
These results raise the issue of which particle size measurements are required to achieve good process control. Here, the in-line analyzer measures the material as it is being produced and can show whether operation is at steady state or not. It cannot however, in this initial configuration, size the discrete microspheres. Is this important?
The answer depends on how the exit stream will be used and which parameters directly correlate with product performance. If a suitable set point can be determined for the in-line analyzer then it will sensitively detect deviations from this value. In this case, the relationship between discrete particle size (which is more likely to be the “critical quality attribute”) and in-line results will depend on the tendency of the material to agglomerate. As previously noted, the degree of agglomeration is known to depend on oil loading but other factors may also be influential. The correlation between discrete particle size and in-line measurements is not therefore straightforward and hence there is a need for pilot scale exploratory work to provide the correct solution.
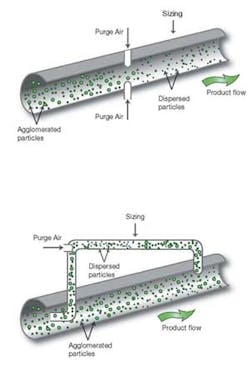
Figure 4: Configurations for in- and on-line dispersion.
One option is to incorporate a dispersion system, in the process line, immediately before the measurement zone (Figure 4). A correctly set air flow will bring the at- and in-line data into close agreement. Clearly, however, this would have an impact on the entire process flow. Alternative approaches include periodic dispersion during measurement only, or a sample loop with purge air—effectively an on- rather than in-line system (Figure 3). The best solution is process dependent, influenced by the scale of operation and the primary requirements of the application.
An alternative method is to mathematically manipulate the results to give the required information. Using data analysis techniques, the measured distribution could be split into two separate modes to provide discrete microsphere size measurement and insight into the degree of agglomeration. If particles above a certain size can be reliably classified as agglomerates then the data acquisition range could be modified to exclude them.
In Conclusion
This experimental study confirms the suitability of laser diffraction particle size analysis as a PAT option for the continuous monitoring of spray drying processes. The particular application—the production of oily microspheres—is demanding because the product tends to agglomerate. Various options are presented to show how sample dispersion can be incorporated into at-, in- and on-line configurations to ensure the accurate size measurement of discrete particles.
With continuous analysis the operator can control the process in a highly responsive way since problems, and the effect of any actions, are immediately obvious. Hence, this enabled the reduction of plant start-up times from ten to two minutes, because the point at which steady state operation was reached could be accurately determined rather than estimated. This reduction decreases waste and increases plant throughput.
More generally, using the appropriate PAT instrumentation enables detailed insight into a process, providing a basis for optimizing product, processing plant and operating practice. The current trend towards increased use of PAT at the pilot scale will give developers rapid and effective access to the information required for QbD and help begin the challenging process of transforming pharmaceutical manufacturing.