In this year’s 6th Annual Report and Survey of Biopharmaceutical Manufacturing, we evaluated the critical areas involving downstream processing and tracked trends impacting the industry. The current study provides a global view from executives at 446 biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) from 35 countries.
Downstream processing is increasingly seen to be a capacity constraint for many companies. This year, 54% of respondents indicated downstream processing was more than a minor bottleneck on overall capacity. A top concern of the industry for many years has been the cost of chromatography steps for purification. In part, this concern is driven by internal company pressure to reduce operating costs and improve product margins: chromatography resin is a large expense. Though we see more specialty resins, including product-specific synthetic affinity matrices, these tend to be priced near those materials they are meant to replace, such as Protein A.
The technical change that has led to the bottleneck in downstream processing is the increase in fermentation and cell culture yields that has been achieved in the industry in recent years. As we double or triple the amount of target protein that is produced in each bioreactor batch, we need to correspondingly increase the amount of target protein we handle in downstream operations. Many expect this trend to continue, which will exacerbate the downstream capacity issues.
Where, then, may we find the opportunities for improvements in downstream capacity and cost? One logical consequence is a desire to move away from Protein A as an affinity chromatography ligand. But there are few potential solutions to increasing capacity and lowering costs of downstream processing. There continue to be few real options open for breakthrough technologies today. We can expect to see incremental improvements in chromatography but not a doubling or tripling of capacity to match the upstream changes.
There are many opportunities for improvements as we face the challenges in downstream processing, but there are few prospects currently for major technical advances to solve our problems.
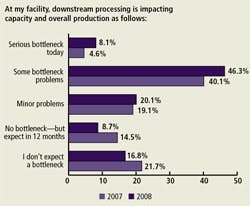
Impact of Downstream Processing on Capacity (Figure A)
More than half (54.4%) of our respondents indicated that their facility experienced “some” or “serious” production and overall capacity bottlenecks due to downstream processing. This is up from last year, when 44.7% expressed such concerns.
U.S. and Western European respondents had different levels of concern regarding this issue: 48.4% of US respondents’ facilities had experienced “some” or “serious” production bottlenecks due to downstream processing issues. In comparison, 62.9% of Western European respondents’ facilities had experienced “some” or “serious” production bottlenecks due to downstream processing issues. And serious bottlenecks were reported by 13% of European respondents, compared to only 2% of U.S. respondents.
(Click to enlarge image) Figure A. Impact of Downstream Processing on Overall Capacity
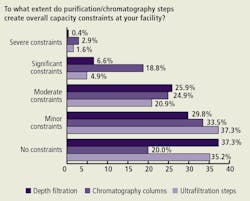
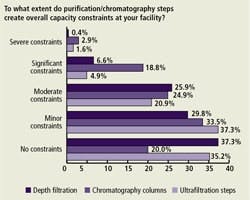
Specific Purification Step Constraints (Figure B)
Relatively few respondents reported experiencing “significant” or “severe” capacity constraints due to depth filtration and ultrafiltration issues. However, 21.7% reported experiencing “significant” or “severe” capacity constraints due to chromatography column issues. All three purification steps were seen as imposing “moderate” constraints by 20-25% of respondents. This is broadly in line with last year’s responses.
(Click to enlarge image) Figure B. Impact on Capacity of Purification Steps
Downstream Purification Issues Facing the Industry Today
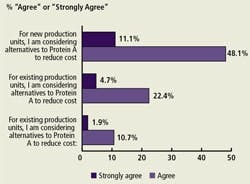
Protein A and Alternatives (Figure C)
We asked respondents to address downstream purification factors affecting their production. We found that an increasing percentage of respondents are considering lower cost alternatives to Protein A for new production units (59%).
(Click to enlarge image) Figure C. Issues Regarding Protein A in Downstream Purification
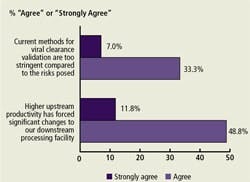
Downstream Purification and Productivity (Figure D)
Most respondents (60.6%) indicated that “higher upstream productivity has forced significant changes to our downstream processing facility.” Only 40.3% believed that current methods for viral clearance validation were too stringent.
(Click to enlarge image) Figure D. Issues Regarding Productivity Impact on Downstream Purification
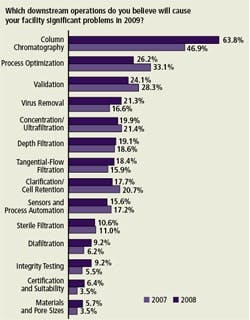
Emerging Problems in Downstream Purification (Figure E)
Downstream bottlenecks are a major concern for many biopharmaceutical manufacturers today. Column chromatography — its costs, cleaning, validation and operation — was once again identified as the lead problem area by respondents, this year by a very wide margin over other potential trouble spots.
Perhaps due to column chromatography’s rising significance, some other contenders appeared to recede slightly in importance, including process optimization and validation. Consistent with other findings, we see that column chromatography continues to grow as a problem. This year, we found 64% of respondents felt column chromatography will create significant problems in 2009. This compares with 47% last year. We measured 13 other factors and found most were relatively similar to last year’s responses.