The production of effective pharmaceutical products that are free from harmful contaminants depends on the ability of the manufacturing facility to maintain an aseptic environment. Whereas some drug products are sterilized by heat or gas at the end of their production, this approach can be damaging for biological products. Instead, the quality and safety of biological products is achieved through tightly regulated aseptic environments that eliminate contamination risk throughout the manufacturing process. Such highly controlled environments must be kept to consistent standards that prevent contamination at every step, from aseptic gowning procedures to the prevention of airborne impurities. Accordingly, regulations and tools have been developed to provide an aseptic manufacturing environment, including current good manufacturing practices (cGMP) and biological safety cabinets (BSCs).
Unfortunately, BSCs were not designed to meet cGMP standards. This can create small windows of opportunity for non-compliance and can pose challenges for those who must ensure BSC performance certification requirements can be integrated into the rigorous cGMP-regulated environment. Pharmaceutical companies are expected to demonstrate that their cabinets provide the appropriate level of protection for the entire duration of drug manufacture, which is not always possible with conventional models. A more meticulous level of monitoring would help ensure processes remain aseptic according to cGMP requirements, and electronically monitored BSCs are well positioned to meet that need.
The disconnect between conventional BSCs and cGMP requirements
Conventional BSCs and cGMP regulations were both developed to achieve different objectives, which has led to a discordance; while the original driver of BSC design was to protect the operator and the environment, sample and product protection is the underlying driver of cGMP requirements. If BSC monitoring is going to be optimized to support cGMP compliance, understanding the difference between these underlying priorities will be critical.
The environment within the cabinet is protected and controlled using high-efficiency particulate air (HEPA) filters. When working properly, HEPA filters enable a precise balance of clean downflow inside the cabinet and inflow drawn from outside the cabinet. Upon installation, fans draw in air which is filtered to provide a particle-free environment for the product, while maintaining a constant protective barrier of air between the cabinet and the operator. This optimal airflow is critical to keeping hazardous products away from the operator, environment and product.
However, captured particles accumulate in the filter over time, resulting in an eventual decline of airflow velocities. These changes will likely remain undetected until the cabinet is re-certified, which occurs every six months for conventional BSCs used in cGMP applications. Often, the service engineer will need to adjust the fan speed or airflow balance for the cabinet to meet certification standards. While this approach may be acceptable in a research environment, it poses an important challenge for pharmaceutical manufacturers operating aseptic processes in highly controlled cGMP environments.
cGMP regulations require manufacturers to demonstrate that their BSCs provide appropriate protection for aseptic processing for the entire duration of drug manufacture and supply relevant written processes controls. Meeting these requirements is challenging, as the need for BSC adjustment upon recertification creates a seed of doubt regarding aseptic conditions. It can be unclear how long BSCs have been operating outside of specifications prior to testing and whether this has compromised the safety and quality of pharmaceutical products. The most common reason for non-compliance is that inflow or downflow was outside of a recommended range, which needs to be adjusted to ensure room air is sufficiently excluded from the BSC work area. Frustratingly, noncompliance with current BSC certification standards is not synonymous with cGMP requirements; at times the cabinet may need to be adjusted, despite the product remaining unaffected.
The design of conventional BSCs is also unsuited to the cGMP requirement of written process controls. While BSCs feature alarms that signal when a remediating action is needed, the operator’s response is not documented. Such records could help provide context to any information that is kept about BSC activity, and could help manufacturers to effectively meet the needs of cGMP environments. To this end, electronically monitored BSCs have been designed with cGMP requirements in mind. More meticulous surveillance, enabled by electronically monitored BSCs, would help manufacturers protect their biological products by enabling a more timely identification of failures and out-of-specification operations.
Electronically monitored BSCs
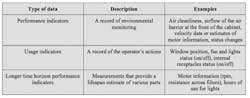
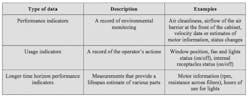
Incorporating electronically monitored BSCs into a cGMP environment requires a different approach to what has been applied in the past. While conventional cabinets alert operators to changes that must be made immediately, electronically monitored BSCs have advanced data collection capabilities that can be harnessed to assure ongoing performance. Electronic monitoring can provide a more insightful record of activities, trends and performance indicators. These records could be exported to demonstrate compliance or to help with the early identification of parameter drift. There are three main types of data that can be collected by electronically monitored BSCs, as outlined in Table 1.
Meeting the needs of cGMP-compliant facilities
Of the three main parameter types that can be continuously tracked through electronic monitoring, each will have particular importance at different stages of processing. For example, performance indicators related to environmental monitoring will be of particular interest to pharmaceutical manufacturers during aseptic phases of production.
Electronic monitoring can improve a manufacturer’s ability to identify and correct failures that could impact product quality. In contrast to the conventional approach where potential issues are identified before too much damage has occurred, electronic monitoring can help to pinpoint when the BSC moved out of compliance and determine what the consequences might be to product quality. There are some situations where a service engineer could discover an issue that doesn’t have any likely consequences for cGMP (e.g., a small exhaust filter leak), yet recommends a repair regardless. A better alignment between cGMP regulations and BSC (and its related work area) monitoring would provide a simpler compliance process.
Better predictions of the need for adjustment and maintenance could have further benefits for pharmaceutical manufacturers; with an extended period of certified compliance, continuous electronic monitoring could reduce the frequency of certification. Anticipating when various elements need to be adjusted or replaced can help manufacturers enjoy certified compliance for longer. Tracking cabinet use can help to identify wasteful and ineffective operations – and even reduce energy costs for the BSC by over 50%. Certain vendors are highly aware of the potential benefits of electronic monitoring and are available to help pharmaceutical manufacturers tailor their documentation to aid regulatory reporting.
Looking ahead
Pharmaceutical manufacturers are required to demonstrate that their BSC – and the surrounding work area – provides an appropriate level of protection for aseptic processing. Electronically monitored BSCs are well positioned to help manufacturers fulfil their requirements, while simplifying the compliance process.
In contrast to the conventional misalignment between BSC and cGMP priorities, modern electronically monitored BSCs can be designed to specifically support cGMP regulations. While there is a significant opportunity to reconsider approaches to certification, it may be difficult to navigate the large volume of data that BSCs can offer. The greatest challenge and value lie in identifying how to use the connected data surrounding usage and performance to streamline and optimize processes.