More pharmaceutical manufacturers are embracing the principles advanced by the Toyota Production System (TPS). Recently, Novartis made the bold claim that it plans to become the Toyota of the pharmaceutical industry (www.pharmamanufacturing.com/articles/2007/088.html).
However, drug makers’ efforts so far seem to focus on only one pillar of TPS: Kanban, or just-in-time production. Far less developed are initiatives around TPS’ other pillar: Jidoka. Often described as automation with a human face, or “autonomation,” Jidoka allows the control system to supervise manufacturing, stopping any process immediately once a problem or error is detected. This practice speeds up root-cause investigation and prevents problems from being pushed down the line and into final product.
“There is widespread confusion over how to define Jidoka, says Bengt Stom, U.K.-based pharmaceutical industry segment manager for ABB Robotics. “Some interpret it as simply the ability to stop a system if things go wrong. We prefer the more holistic concept that the automation system is intelligent enough to assess what is going on and act accordingly, as a human being might.”
Jidoka guides process control practices in a number of industries today. However, drug manufacturing is not there yet, according to Bob Honor, vice president of life sciences at Rockwell Automation. “At this point, many pharma companies are still happy to get into production.”
The plastics industry provides great examples for in-line automated quality control, he explains, while semiconductor manufacturers have used these principles for over 20 years. “There are semiconductor facilities with process capability exceeding eight sigma,” he says.
Process analytical technologies (PAT) best embody Jidoka in the pharmaceutical industry today, says Bob Lenich, life science strategic business director at Emerson Process Management. Currently, he notes, a group of industry leaders is vested in these technologies. He foresees that eventually, the industry will rely more on inline analyzers to provide real-time feedback, and the use of applied modeling and simulation to facilitate decisionmaking and closed-loop processes. “PAT advances are now seen most often in the fill and finish portions of manufacturing, with the goal of increasing throughput and decreasing cost of quality,” Lenich observes. “Once an API or biologic product has been produced and needs to be packaged, the Jidoka principles apply very well.”
However, notes Rockwell’s Honor, PAT implementation in pharma is still occurring piecemeal, on a project-by-project basis. Now, most advanced customers are working on infrastructure for PAT, but the next step will be more advanced realtime control and Quality by Design, Honor suggests. Companies are studying advanced process control techniques for manufacturing, but this work is almost “like a research project,” he says and the idea of applying fuzzy logic or adaptive control is still alien to many pharma companies.
As one respondent to our automation and process control survey remarked, “I have tried to implement some of these controls, but our director believes that we should control inputs rather than outputs, the opposite to the approach required by these control philosophies.” Regulatory requirements and enforcement may be part of the problem. The wording of FDA regulations can be interpreted either rigidly or broadly, but the final issue is how individual FDA inspectors are interpreting them. Vendors point to Bristol-Myers Squibb’s new automation and control platform, now being implemented at the company’s new biologics facility in Massachusetts, as an example of the future of pharma, incorporating simulation, PAT and advanced process control. This platform, however, reportedly took several years to conceptualize and build. (An article in September’s issue will examine the installation in depth).
Nevertheless, our survey indicates that pharma is making some progress in using Jidoka. This year, 67 pharmaceutical industry professionals responded to the survey so results may not be statistically significant, but still provide insights into trends. Among the most notable results this year:
- A greater percentage of respondents are implementing PAT, either alone or as part of a broader Quality by Design initiative.
- Alarm management has become a key focus for more respondents. This effort is critical to improving the human-machine interface, a key to reducing human error, a cornerstone of Jidoka.
- More companies describe their IT and process control efforts as being more aligned with each other than they were in the past.
- While most respondents say they do not plan to use robotics, some early adopters are applying robotic technologies to packaging lines and to sample preparation.
- Most respondents say they are not planning to incorporate continuous manufacturing.
Key Process Control Issues for 2007
In pinpointing their biggest process control and automation needs this year, respondents rated the following as “extremely important”:
- Developing trending data for equipment and batch manufacturing;
- Improving alarm management.
Interestingly, this was not seen as a priority by most respondents last year. “Very important” were:
- Improving the quality of data for process modeling and optimization;
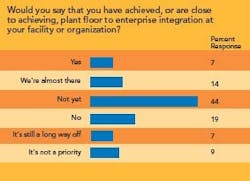
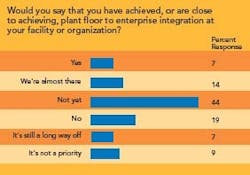
- Integrating maintenance and manufacturing data, as well as plant-floor data, with an enterprise resource planning (ERP) system.
Alignment Improving Respondents noted that they are making progress in aligning overall continuous improvement projects with their IT and process automation goals. About 29% acknowledged that they are moving forward in this area, and 40% describe operations as aligned “to some extent.” However, 30% say their automation and IT projects are not yet in sync with continuous improvement efforts.
Advanced Control Process capability (Cpk) analysis and statistical process control (SPC) are becoming more important to a growing number of respondents to this year’s survey. However, SPC efforts are primarily univariate, rather than multivariate. Whereas 11% of respondents use Cpk and both univariate and multivariate SPC routinely, and 6% say they use these techniques “occasionally,” 28% of respondents say they are not using either analytical method. About 34% say they perform Cpk and univariate SPC routinely or occasionally, and 13% use process capability analysis only. Process analytical technologies (PAT) are being used at more respondents’ facilities.
About 25% of respondents say they are doing PAT projects and plan to implement Quality by Design (QbD). Another 16% say they’re planning to start using PAT but not QbD. Roughly 27% are doing both, and 16% are focusing on QbD and view PAT as part of a broader effort. However, 21% say they aren’t using either approach and have no plans to start. Advanced Control Strategies Less than half of all respondents say they are using more advanced process control (APC) at their facilities. Within that group, 13% use Model Predictive Control, nearly 23% employ fuzzy logic and artificial intelligence, 39% use simulation and 42% utilize other advanced control methods.
Among APC users, 78% are applying the tools to manufacturing. The majority of those employing simulation use it for process optimization; secondary uses include supply chain management and facility design. Wireless and Robotics Wireless appears to be making inroads, slowly, with 37% of respondents saying they don’t plan to use it, but 30% say that although they’re not using it today, they plan to adopt it for warehousing and process applications.
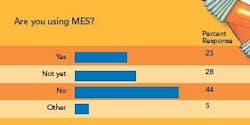
Robotics is also attracting a small group of early adopters — 44% of respondents are not using it and 23% say they do not plan to, but 28% are using it and another 5% plan to. Users say packaging is the top application, followed by sample preparation and palletizing.
Batch manufacturing appears to be a way of life for pharma, with 64% of respondents saying they have no plans to implement continuous processes. However, 29% say they employ both batch and continuous processing at their facilities, and another 7% say they will be adding continuous processing capability in the future.