One would be hard pressed to find an industry more risk averse or slow to change than the pharmaceutical industry often, for very good reason. Certainly, pharmaceutical blister packaging is a part of pharmas conservative culture, and has its own anecdotal lessons as to the consequences of risk and change.
However, there is a need for change. Not only have FDA and other global regulatory agencies called for a new risk-based manufacturing philosophy, but the marketplace is demanding materials with extremely effective barrier properties, as well as new packaging technologies.
Given the trend to use highly sensitive active pharmaceutical ingredients (API) and excipients in drug formulation, and the need to keep packaging off the critical path during the drug approval process, there is a growing need for extreme barriers, or packaging materials with increasingly high barrier properties. Such packaging will also be needed for tomorrows drug delivery systems.
But several challenges must be addressed if these materials are ever to be used routinely in pharmaceutical packaging: the need for technological change, growing demand for extreme barrier properties, and the tension between risk management and risk avoidance. This article will focus on solutions to these challenges, which have already been developed in other industries and now await implementation in pharmaceutical packaging.
Such solutions will need to be science-based and transparent. The future of barrier packaging demands nonproprietary technologies with open standards that assist rapid adoption and risk management. The question is whether the drug industry will embrace this demand, and reap the rewards that come with it.
Extreme barrier packaging
One can often best see the future of a technology or marketplace by examining its cutting edge. One can only understand extreme barrier packaging by understanding materials, machinery and tooling.
As noted above, pharmaceutical blister packaging today generally relies on well-known, well-proven solutions. In extreme barrier packaging, this traditionally means either the 50 µm (2 mil) Aclar structures originally developed for the U.S. market, or three-ply CFF structures originally developed in the European market.
However, users are demanding ever more extreme barriers, especially against moisture.
Three distinct trends drive this demand for more barrier, and will become even more important in the future:
- The growing importance of drug delivery systems;
- The tendency to develop APIs that are highly sensitive to moisture and oxygen;
- Use of super disintegrants such as sodium starch glycolate, which are becoming more important in formulation. These materials are increasingly being used in generic drug manufacturing, to help achieve desired dissolution curves and enhance bioavailability. Unlike traditional excipients, these materials require packaging materials with a much higher moisture barrier.
Until now, this demand for packaging with improved barrier properties has been met mainly by enhancing existing products, or combining materials. Each of the four traditional forming material technologies (CFF, PCTFE, COC, and PVdC) is available in new forms that address the growing demand for extreme barrier properties.
- CFF/Alu Alu: New four- and five-layer structures reduce any risk of delamination and pin-hole, and enhance stiffness and processing speed.
- ACLAR/PCTFE: Films are now available up to 100 µm or 4 mils thick, doubling the barrier offered in the traditional 50 µm or 2-mil structures.
- TOPAS/COC: New combinations of COC with PVdC are pushing barriers obtainable with these structures into barrier ranges formerly available only from CFF/Alu Alu or Aclar/PCTFE.
- PVdC: Solvay is working on a new product with several of its converters that it says significantly enhances the barrier obtained from a given gram coating weight.
In the future, extreme barrier packaging materials will most likely further segment depending on how barrier is achieved. The two current barrier alternatives, metallic (aluminum) or polymeric (PCTFE, COC or PVdC), will each continue to follow their own paths.
In metals (CFF), expect to see a focus on:
- Structure enhancement: Here, the first question will be: which polymeric film, if any, will best optimize aluminums ability to form optimal cavity geometry without stress fractures, delamination, or pin holes? The second question will be how to increase processing speed.
- Aluminum enhancement: In the future, manufacturers will routinely evaluate the ideal gauge of the aluminum substrate, and use metallurgical advances to optimize the aluminum structure.
In polymeric films, such as those using ACLAR/PCTFE, increasing attention will be paid to:
- Use of Orientation: Mono and bi-axial orientation will be used to further enhance barrier properties.
- Structures: More combinations of materials and thicknesses will be evaluated, to further enhance barrier properties.
No more one size fits all machinery and tooling
In the recent past, machinery suppliers marketed blister packaging machines that were versatile enough to to process a variety of materials, including CFF, ACLAR/PCTFE, TOPAS/COC, PVdC, even polypropylene (PP). Many equipment manufacturers developed machines that could process both cold and thermoformable structures.
Cold forming: In order to gain the geometries required to provide extreme barrier, CFF is poised to change the way that structures are formed. Existing metal forming technologies from other industries, if migrated to pharmaceutical machinery, offer the potential to achieve the geometries, speed and security demanded by the industry. Major changes can also be expected in the design and materials used to make plug assists or forming pins that will enhance the ability to form the structure, and allow for more radical shapes with less risk of pin-holes, delamination and stress fracturing.
Thermoforming: Here, technology is also poised for major technological changes. In order to achieve better material distribution and thus optimize barrier, new technological advances in the way materials are heated and formed are being developed for pharmaceutical packaging.
These technologies will allow easier, faster and more efficient processing of extreme barrier materials. The recent introduction of new materials such as copper beryllium in the pre-heating technology is an example of what is to come. More radical solutions include zoned heating, as well as alternatives to the plate heating systems that the industry has traditionally used since the introduction of blister packaging.
Tooling: Both cold forming and thermoforming for extreme barrier will require high-tech tooling. Changes will be needed in:
- tooling design;
- materials used to make tools (especially plug assists and forming pins);
- technology used to manufacture tools at the tolerance needed for sophisticated, extreme barrier applications.
The ability to qualify tooling may also change the future of how tooling is supplied.
Traditionally tightly controlled by the machinery manufacturer, tooling technology from other industries, such as automotive or aerospace, offers significant enhancements in extreme barrier, ease of design and performance. Such tooling may segment the supply of blister tooling from its traditional close connection with OEM blister machinery suppliers.
Active barriers
Another area offers the potential for major change in the future of extreme barrier blister packaging: active barrier. While alternative container systems, especially bottles, have used desiccants and scavengers to actively provide extreme barrier, blister packaging lags behind on this front.
The driver, again, will be the need for extreme barrier in this case, a blister structure that is capable of actively eliminating moisture or oxygen. Right now, there are various contenders for an active barrier technology for blister packaging:
- Desiccant can be contained in the blister itself with channels to allow the material to scavenge moisture or oxygen, actively;
- Nanotechnology can be used to develop films that will scavenge moisture or oxygen;
- Active barrier can be added to the lidding stock.
For the time being, though, the need for an extreme, active barrier in pharmaceutical blister packaging has yet to be met.
Sealing
An extreme barrier pack requires an extreme seal. Current developments in sealing technologies are beyond the scope of this article, but the following hold true:
- By definition, an extreme barrier package requires extremely good seals;
- The industry standard for testing seal hermeticity, the infamous methylene blue test, cannot demonstrate, adequately, the kind of sealing required by an extreme barrier package.
Beyond stability testing
The cost of stability testing is high, both in terms of the time and the accuracy required. One of the most significant questions in the future of barrier blister packaging is whether alternative technologies can reduce the current dependence on stability testing results, given their high costs and long lead times.
Forward thinking pharmaceutical companies are already examining how to use barrier packaging to reduce stability testing overhead in two separate arenas: pre- and post-approval.
Pre-approval
In the pre-approval world, if barrier packaging can reduce the time to approval, it will benefit the company, either by accelerating stability testing or enhancing the probability that the package will pass its first time through.
There is a strong demand for barrier packaging that either speeds launch times or reduces the risk of a stability failure. The tendency to use high barrier packaging to speed approvals will continue to increase into the foreseeable future. In fact, many companies are already selecting extreme barrier materials as launch structures, in order to reduce the risk of packaging failure during an accelerated stability test.
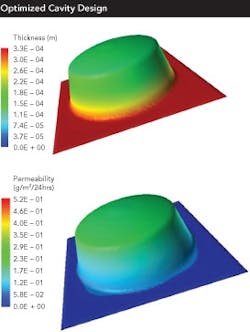
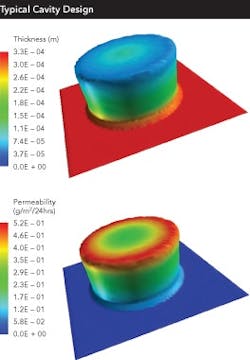
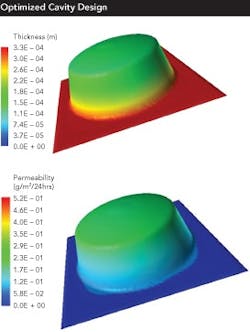
One major recent change will become increasingly important to the future of barrier packaging: the measuring and tabulation of the formed barrier of a given cavity geometry/material combination. This is a major change from traditional stability testing, which only provides a pass-fail data point at the end of a minimum of six months of testing.
At the forefront of these changes are those companies that are now using USP 671 to link specific barriers to specific cavity designs and material combinations. The challenge to this methodology is the amount of work and time involved.
However, two new technologies offer the possibility of a much faster solution: Finite Element Analysis (FEA) can be used to simulate the barrier achieved by a specific cavity geometry and barrier material choice. Microtome analysis can be used to study a formed blister.
FEA uses computer software to simulate the formed barrier of a given geometry/material combination. Microtome then slices a formed blister and allows easy and precise measurement of the barrier layer throughout the blister.
The combination of FEA, Microtome and in-house USP 671 testing can offer a pharmaceutical company the chance to determine its confidence in this new technology. In the future, companies may use this technology to best choose an extreme barrier material for pre-launch stability testing.
Post-approval
There is an enormous global pressure to reduce costs in post-approval packaging. Once the product is approved, barrier materials represent a cost, and reduced margins, thus more companies are emphasizing the importance of lowering post-approval packaging costs.
The first strategy that companies used to help reduce barrier packaging costs was using global tenders, often in the form of online bidding on the Internet. This approach has a distinct disadvantage: at some point, suppliers of specialized products that are difficult to make, and will never reach commodity volumes, opt not to bid. Quality and service can be affected, and the vendor/supplier relationship is strained.
Another, better way to lower post-approval barrier packaging costs is through design optimization. Recent technology reveals something that the industry has long suspected: cavity design, tooling and processing can have as much and sometimes more of an impact on the formed barrier provided by a specific cavity than the barrier material itself.
Fundamental design issues include:
- the draft angles employed
- the use of radius vs. chamfers, flat vs. curved bottoms
Each issue can enhance or degrade barrier by a significant amount. And, since stability tests are pass-fail methodologies, they do not provide data on the actual barrier of a given cavity geometry in a given barrier material.
FDA's Guidance for Industry on Changes to an Approved NDA or ANDA, issued in November of 1999 and revised in April 2004, defined a change in blister packaging of an oral solid dosage form as a minor change, which could be listed in the annual report without prior approval. The stability requirement could be fulfilled with the annual stability put-up, thus eliminating an incremental stability test. This held true as long as the new package provides the same or better protective properties and any new primary packaging component materials have been used in and been in contact with CDER-approved products of the same type.
This opens up large potential cost savings for the pharmaceutical industry. FDAs guideline established the same or better protective properties as the bar for effecting a change without the need for prior approval.
The question that needs to be answered in the future is whether the technology noted above, the use of FEA simulation and Microtome measurements, can provide robust data that demonstrates the same or better protective properties. If so, companies will be able to optimize the design of tooling to achieve the same barrier from a less costly unformed film. If a design change provides the same barrier in the formed cavity using a significantly less costly base film, a true win-win scenario can be envisioned.
In short, it seems likely that the use of FEA simulations and Microtome analysis to reduce the delays and costs inherent in stability tests will accelerate the use and application of barrier materials. Moreover, this same technology can offer a major strategy to reduce costs in post-approval products.
Change will gradually accelerate in the pharmaceutical barrier blister packaging market. The use of barrier packaging materials will continue to grow, driven by product requirements and speed-to-market considerations. Extreme barrier materials will mature. Barrier material structures may see incremental change at best, but machinery and tooling will be transformed, driven by new technologies and ever tighter performance requirements.
One potential area for major change is active barrier, but there is no clear technology at this date offering a complete solution. Even if it existed, adoption might be faster than prior technologies, but would still take time.
Whether the pharmaceutical industry will adopt new virtual technologies such as FEA simulations, Microtomes and 3-D rendering to analyze and verify barrier packaging remains to be seen. Such technologies have the potential to eliminate delays and significantly reduce the costs associated with stability testing. They offer users the opportunity to use the best available technologies for risk management, and allow for a major shift from supplier-driven to user-driven barrier packaging technologies.
About the Author
Peter Schmitt founded Montesino Associates in July 1996 to provide marketing services for companies in the plastics and packaging industries in the Americas. Schmitt began his career selling polymeric raw materials to the medical, pharmaceutical and electronics industries. His company was acquired by a division of General Electric. Schmitt later was director of marketing for American Mirrex, a manufacturer of PVC film for the pharmaceutical and medical device industries. Schmitt has a M.Div. from St. Bernards and a B.A. from St. John Fisher.