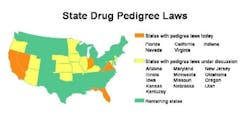
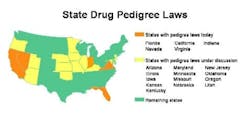
Florida is leading all states the stringency of its drug pedigree laws. Florida law, effective July 2006, will require pedigrees for all drugs, meaning products must be authenticated and certified preferably by electronic means by all wholesalers, repackagers and pharmacy distribution centers. Retail and institutional pharmacies will receive and retain the pedigrees for drugs they dispense.
Many states plan to follow Floridas draft rules to automatically authenticate and certify electronic pedigrees. This can be done by using industry-standard security technologies such as PKI (public key infrastructure) and digital signatures. Seven states have passed pedigree legislation, and pedigree bills have been proposed in another ten (see map, below).
Californias pedigree law, which will take effect January 2007, requires manufacturers, as well as wholesalers, pharmacies and repackagers to maintain pedigrees. Since California and Florida are the largest drug markets, most U.S. wholesalers and pharmacies are expected to provide electronic pedigrees by July 2006 in order to distribute into these two states.
Within the next year, manufacturers should serialize their drug products with either barcodes or RFID and work with their supply chain partners to ensure their inventories have pedigrees. Most, if not all, trading partners will have pedigree-related safe and secure solutions in place.
With so much variability in legislation between states, calls have been made for a universal pedigree initiative and legislation.