Industry after roiled industry is being transformed as digital innovation becomes the mother of new methods, efficiencies - and businesses.
In pharmaceuticals, the first wave of the digital revolution has largely been focused on using technology platforms and data analytics to better understand customers, increasing patient engagement and developing new go-to-market approaches.
But advances in the pharmaceutical Internet of Things, sometimes startling in their simplicity, are speeding up the pace of change as it becomes increasingly evident that many of these innovations can give companies an edge in productivity, delivery time, quality control and planning. Ignoring or not embracing them will put a company at a competitive disadvantage to those players who do embrace innovation.
For executives overseeing pharmaceutical operations, staying abreast of the breakthroughs that are here, just ahead and a little further down the road can be daunting as they decide how to apply digital technology to today’s challenges.
Still, it is imperative to be bold in adopting innovations and building a digital supply chain ecosystem that creates seamless interactivity between humans, machines and resources.
The key words - and goals - are connectivity and transparency.
Driven by digitization, cloud-based connectivity and visibility can mean faster and better informed decision-making and more accurate planning as sensors built into the supply chain deliver real-time data on an ongoing basis.
Globally available transparency helps to optimize manufacturing and maintenance while reducing downtime as machine-to-machine communication and machine-learning algorithms allow for seamless processes, predictive servicing of equipment and automatic corrective actions. Automation and digitization can also deliver major efficiencies in procurement, logistics and warehousing.
Importantly, true visibility allows for greater end-to-end supply chain integration and network scalability since cloud-based networks make it much easier to link all players - everyone from suppliers of raw materials, contract manufacturers and third-party logistics providers to wholesalers and distributors - in a single integrated network. Fully integrated planning processes become a reality - allowing for higher consistency, accuracy and, with that greater alignment, shorter lead times. Together, such advances can have a positive impact on working capital.
How do you realize these heady promises? How do you introduce the transparency that will streamline operations and ultimately improve financial performance?
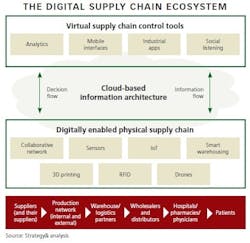
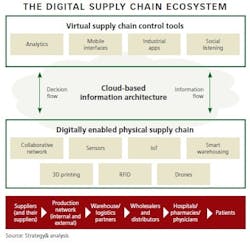
The first step is to create a supply chain ecosystem that has three essential layers:
1. Virtual Tools
Software that enables mobile, collaborative and dynamic decision-making allows managers to oversee and control operations across the entire supply chain. Data from all points of the supply chain is processed by analytics tools and is accessible via computers and mobile devices. Industrial applications and optimization tools run analytics, generating insight on all aspects of the supply chain, such as demand/supply orders; inventory levels; goods in transit; manufacturing performance by plant, line, and individual machine; utilization; and many other metrics that facilitate real-time decisions. With these tools in place, managers have full visibility of the supply chain.
2. Cloud-Based Information Architecture
Cloud computing is the backbone of the digital ecosystem, and its flexibility allows it to work across different methods of data origination and systems. It can be layered over existing ERP systems at various nodes, allowing for easy integration and scalability. Collecting, translating and storing all data in the cloud makes it accessible to everyone at any time and at any point/node in the supply chain.
3. “Smart” Digital Components
Imagine factories with manufacturing lines containing thousands of sensors that track and report performance so adjustments can be made. In warehouses, scanning drones, packing robots and automatically guided vehicles perform highly automated work. Materials and products transmit their location through the network. Smart storage cabinets and refrigerators in hospitals and pharmacies track and report consumption data and replenishment requirements. In this third layer of the ecosystem, the physical supply chain - the pharma Internet of Things - actually comes alive via sensors, RFID tags, wireless transmitters and other technologies.
When all three layers work in a synchronized way, cyber-physical systems are formed, with humans, machines and resources communicating as in a social network. This fundamentally transforms the supply chain from today’s mostly segmented/siloed structures into truly integrated, interactive systems.
Certainly, digitizing operations is not an overnight proposition. It is a complex task that requires companies to develop solutions not within their own four walls but in alignment with the many external partners along the supply chain, keeping in mind that technological advances can make digitization a moving target. To meet this challenge, companies will need to break out of entrenched ways of working and overcome resistance from organizations and employees accustomed to processes in place for decades. Moreover, there is a good deal of hype to cut through and risks to manage. And regulatory aspects add an additional layer of complexity — not only will this be a new paradigm for companies but also a dynamic environment to which regulators have to adapt as well.
There are several key success factors on this journey. Companies need to start with a clear understanding of the ecosystem and evolving technologies. They also must have the right resources in place, including a cross-functional team of experts and the requisite capital to make needed investments. Collaboration with partners is crucial if players are to develop truly integrated end-to-end solutions. And - critically - companies must have a mindset of experimentation and learning by doing to build the necessary “digital capabilities.”
At the same time, pharma companies will need to manage several key risks and concerns. The biggest is cybersecurity. The threat of hackers getting control over digitized physical assets and machinery is a more real threat than ever and every precaution must be taken, every firewall available built. Similarly, ensuring secure flows of sensitive data on the cloud (including demand, supply, pricing and contract information) is a baseline requirement that cannot be emphasized
Also, with such a wide range of technologies and rapid development cycles, the pharmaceutical industry will require some level of standardization to ensure interoperability among different systems and devices.
Those companies that seize the initiative can realize a sustainable competitive advantage; operate with greater agility, cost-efficiency, and control; and ultimately provide better care for patients.
ABOUT THE AUTHOR
Dr. Marcus Ehrhardt is an advisor to executives on life sciences strategy with a special focus on strategy-based transformations. Based in New York, he is a leader in the life sciences strategy business within Strategy&, PwC’s strategy consulting group. He is a principal with PwC U.S.