Transforming Bioburden Risk with Digital Asset Intelligence
The concept of a “smart asset” means different things to different people, but the way we think of it is simple: an asset is able to add business value by telling its story, digitally, to anyone with a smartphone-based reader and proper security credentials. In many ways, smart asset technology is a matter of “RFID redefined.”
In the context of aseptic pharmaceutical manufacturing, the smart asset approach serves a dual role for risk management: 1) it allows for automated, touchless environmental monitoring to support sterilization surety during production; and 2) it provides traceability and pedigree data from sterile processing through manufacturing to support FDA regulated facilities so that products can be released to inventory at a higher frequency, and with minimized risk due to contamination.
DATA ON AN ASSET'S PHYSICAL LAYER MATTERS
Aseptic manufacturers are already required to deliver meaningful information about the quality of the processing environment; they must demonstrate to regulators that proper controls are in place, and they must retain the right data to support root cause analysis in the event of a downstream recall. Yet they still face challenges to a) gather this data, and b) do so in a way that minimizes the potential for human-caused contamination in the sterile environment.
To address this issue, the simple step of putting digital data directly onto the physical components that must be sterilized reduces the number of human touches, provides a digital pedigree of manufacturing processes and stages and thus limit the chance that a contaminated environment may lead to a flagged or wasted production run.
A SHIFT IN IOT MINDSET: FROM “I” TO “T”
How do you turn physical assets into compliance and integrity oversight devices? Advances in computer miniaturization and the steady march of Moore’s Law have made it possible for rugged semiconductor chips to be attached to, or embedded within, any given sterile asset. These data-carrying chips require no batteries or wired connections — instead, they harvest their power from radio frequency (RF) signals that interact with the asset when communicating with it.
In a pharmaceutical manufacturing environment, the intelligent assets we’re talking about are varied. They could include biologic collection containers and packages, or the myriad of components used to monitor airborne particulates, active viable air, passive viable air, equipment surfaces and facility personnel themselves. Whenever a drug or biologic goes through its given process or stage of production, the components gather digital records and time-stamped details about the manufacturing stage, location or condition of the environment, which can include chain-of-custody and integrity information needed for regulatory compliance. These assets become the literal digital thread for the regulatory and compliance database that helps personnel perform their jobs better and improve outcomes. Operators, laboratory technicians and managers can digitally access and sync component data to bring about better documented production outcomes and safer drugs released into the marketplace.
Granting an asset better intelligence (or data) at its physical layer is no doubt a novel approach, a departure from typical sensor-based IoT thinking that centers upon the “I” (or, connected) part of the IoT. It is our belief the focus has skewed far too heavily toward promoting a need to connect everything with a sensor, all the time, and then streaming the information to the enterprise cloud. When you start having to account for the variables and infrastructure required to maintain always-on connectivity, the value proposition for the solution gets lost amidst very real expense-to-return ratio concerns.
However, when you flip the mindset toward putting reliable, rugged, compliance data on assets themselves, not only do you remove the necessity for a corporate-wide, networked software environment, but you open the door to new workflow efficiency from unexpected places. For example, today’s barcoding standard requires tracking each asset individually, which relies on frequent scanning, necessitates manual intervention by work crews and becomes a bottleneck to productivity. With the asset intelligence approach, however, there’s significant work reduction within the touchless process itself. Instead of scanning individual containers, one at a time, it is now possible to gather all manufacturing and product information with much less operator interaction. This results in measurable improvements by a factor of 20: Not 20 percent, but a reduction to one-twentieth of previous time and effort required. A shipment that used to take eight hours to process with barcodes can now be received and catalogued in roughly 30 minutes. The expense-to-return ratios are more palatable.
WHERE ASSET INTELLIGENCE COMES TO LIFE
Getting data onto assets is a relatively easy concept to grasp. The aseptic industry understands, however, that any data traveling with a biologic product or monitoring an injectable drug through manufacturing must be able to survive exposure to radioactive sterilization processes. Without a doubt, there’s a long-held understanding that electronic radio-frequency data is unable to maintain its stability and reliability when exposed to harsh sterilization such as gamma rays and eBeam. Fortunately, asset intelligence has upped its game on this front.
Technology advances have enabled not only gamma and eBeam sterilization-proof semi-conductor chips and tags, but also digital data memory storage, retention and file management capability far exceeding the 128 bit limits of traditional RFID. In fact, these advances now deliver 32 Kbytes of storage with more than 500 Kbyte total capacity using compression algorithms. Asset intelligence includes other novel features such as encryption of digital signatures, public key infrastructure (PKI) and digital memory partitioning (so that certain data can be selectively available to users based on their administrative rights and roles). In reality, this “new” technology is actually not so new; it has been used to improve complicated and critical aerospace supply chain and maintenance operations for the last six years. But the reliability of the data and its imperviousness to extreme manufacturing processes — as is mandatory to sterile manufacturing — has brought the solution into the pharmaceutical limelight. As it stands today, it is the only viable solution ideal for this industry’s challenges.
THE SELF-CONTAINED RECALL IN BIOLOGIC MANUFACTURING
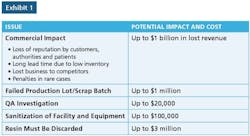
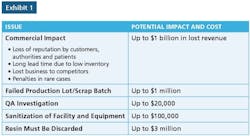
Microbiologic-related recalls have made up a significant portion of the enforcement actions by FDA for many years. More than 75 percent of FDA recalls from the years 2004-2011 involved sterile products, and about 80 percent of these recalls were linked to “lack of sterility assurance.”
Since these odds are so high, it stands to reason that biologics manufacturers need to make processes and its manufacturing containers digitally traceable from time of collection through to final production. For example, let’s say you have 20 million collection containers stored across multiple, global facilities - in a variety of pre-production stages - including cold storage. The system must be able to locate and surgically extract a single recalled container without disrupting the entire business operation.
When detailed information about a product’s manufacture, its chain of custody, its travel, storage and current location is tied to the product itself, it can quickly and selectively be flagged for removal. This is a critical value-add in the matter of streamlined recall management and reducing waste.
TOUCHLESS ENVIRONMENTAL MONITORING
Of course, recall events aren’t good for anyone, and the goal is to prevent them in the first place. When we see the above statistic that 80 percent of microbiologic recalls are linked to a “lack of sterility assurance,” it tells us current processes need to be better at monitoring sterility.
By enabling sterilization-proof electronic data to be written directly onto environmental monitoring equipment, and collected digitally at multiple prescribed points throughout the production process, cGMP manufacturers can see dramatic improvement in their productivity, while yielding more accurate and thorough data collection for compliance reporting and recall containment.
Ultimately, asset intelligence gives cGMP manufacturers a new way to harness the information they need to prove manufacturing compliance for zero contaminants. Beyond important fiscal and brand protection reasons, stringent control of aseptic processes is in place for a larger reason: to avoid unwanted, unsafe patient consequences. True process control is best achieved through careful analysis, holistic understanding and thoughtful design. However, none of these aims can be achieved without proper access to data. When assets become intelligent enough to carry and deliver data to the people who need it, at the exact time they need it, there’s a transformative effect for all involved.
REFERENCES
David Westman, GE Healthcare, Bioprocess Online, May 3, 2017.
S. Sutton and L. Jimenez, American Pharmaceutical Review 15 (1), pp. 42–57 (2012).