US is dangerously dependent on China, India for key starting materials
The U.S. supply chain for key starting materials (KSMs), the building blocks needed to manufacture active pharmaceutical ingredients (APIs), is at risk due to America’s reliance on countries such as China and India for these critical chemicals. That’s the finding of nonprofit organization US Pharmacopeia (USP).
“KSMs are the foundational chemicals necessary to commercially synthesize APIs,” according to USP. “Understanding trends like where KSMs are made, and where they are solely sourced from a single jurisdiction, can help decision-makers take action to address vulnerabilities in our supply chain.”
While there has historically been little insight into the upstream supply chain for medicines, USP claims that its manufacturing scientists have identified all KSMs likely used in the commercial production of all U.S.-approved APIs.
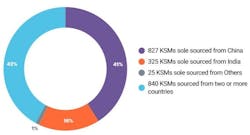
Based on their data, USP found that 58% of KSMs used for U.S.-approved APIs are sole sourced from a single country, with 41% of such KSMs from China and 16% from India. At the same time, the analysis showed that 17% of U.S.-approved APIs rely on a single country for all their KSM sourcing.
USP’s analysis also revealed that 50% of U.S.-approved APIs rely on a single country for at least one KSM, with China being the sole supplier of at least one KSM for 679 APIs, representing 37% of all APIs, while India is the sole supplier of at least one KSM for 402 APIs and accounting for 22% of all APIs.
USP cited amoxicillin, the second most prescribed oral antibiotic in the U.S., as an example of America’s dangerous dependence on China for KSMs. While numerous manufacturers globally produce amoxicillin API and formulate it into finished oral dosage forms, with some of the intermediate KSMs made in India and elsewhere, its synthesis ultimately depends on four KSMs — each produced almost entirely in China — according to USP.
“The most critical, 6-aminopenicillanic acid (6-APA), is also essential for other penicillin-based antibiotics such as Ampicillin, Dicloxacillin, Nafcillin, Oxacillin, and Piperacillin,” USP warned. “As a result, China effectively holds the key to amoxicillin production, and the production of several other essential penicillin-based antibiotics. Any disruption — such as a factory shutdown or export restriction — could quickly cascade through the global supply chain.”
Advanced manufacturing technologies are needed to rebuild America’s domestic production of APIs and KSMs, according to USP, which called on Congress and federal agencies to address these sourcing vulnerabilities. Incentivizing the acceleration of innovative approaches such as continuous manufacturing and flow chemistry are essential to modernizing, localizing, and stabilizing U.S. production, the group said.
“New approaches like alternate synthesis pathways and innovative chemical processes, as well as advanced continuous flow manufacturing, can help make production economically viable at scale,” contends USP. “These approaches strengthen rapid response capabilities, reduce supply chain vulnerabilities, and enhance our ability to safeguard public health and national security.”
What about auxiliary chemicals?
KSMs are needed to manufacture APIs, which in turn are necessary — along with excipients and other materials — to make a finished drug product. For small molecule drugs, API production includes a multi-step process that starts with the preparation of KSMs and performing controlled chemical synthesis through a series of reactions that use auxiliary chemicals.
In a July 28 analysis, Brookings Institution senior fellow Marta Wosińska and senior research assistant Yihan Shi discussed the critical role China plays in auxiliary chemicals, such as the production of reagents and solvents.
“These chemicals are necessary for the chemical synthesis of API and intermediates yet are omitted from virtually all analyses,” Wosińska and Shi wrote. “Auxiliary chemicals should be included in vulnerability assessments for essential medicines and considered in any stockpiling programs of precursors.”
They concluded that “onshoring active ingredient production will not address the risk unless access to raw materials is also derisked” and “policy interest should be broadened from KSMs to auxiliary chemicals that are needed in the chemical synthesis of API or as excipients.”
About the Author
Greg Slabodkin
Editor in Chief
As Editor in Chief, Greg oversees all aspects of planning, managing and producing the content for Pharma Manufacturing’s print magazines, website, digital products, and in-person events, as well as the daily operations of its editorial team.
For more than 20 years, Greg has covered the healthcare, life sciences, and medical device industries for several trade publications. He is the recipient of a Post-Newsweek Business Information Editorial Excellence Award for his news reporting and a Gold Award for Best Case Study from the American Society of Healthcare Publication Editors. In addition, Greg is a Healthcare Fellow from the Society for Advancing Business Editing and Writing.
When not covering the pharma manufacturing industry, he is an avid Buffalo Bills football fan, likes to kayak and plays guitar.