Q&A: Addressing Drug Supply Chain Security
To combat potentially unsafe counterfeit drugs and product diversion, the FDA passed the Drug Supply Chain Security Act (DSCSA) in 2013, creating federally mandated requirements for traceability of pharmaceutical products. Fast approaching is a November 2017 DSCSA compliance deadline with additional serialization requirements. While the disruption to existing manufacturing and distribution processes will be significant, it is vital to impose supply chain visibility to prevent diversion and counterfeit products from reaching consumers.
PMMI sat down with Steve Peterson, product manager at the Peterson Group and Mario Simard, senior product line manager at Optel Vision to discuss what strategies pharmaceutical manufacturers should consider, and pitfalls to avoid. Peterson and Simard will present during the Compliance Trends track of the Conference at Pharma EXPO (McCormick Place; Nov. 6-9).
Q: Can you elaborate on the DSCSA compliance that is required by November 2017?
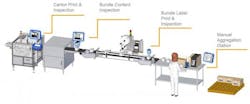
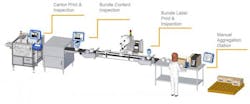
A: DSCSA Compliance is rolled out in three distinct phases for manufacturers over a 10-year span, and the second phase of compliance is due in November 2017. This will require drug product packaging to have unique product identifiers (serial numbers), including the Global Trade Identification Number (GTIN) and a 2D barcode. This information must be printed onto the unit of sale and the case.
Q: When should manufacturers implement a serialization strategy?
A: It is already very late in the game to implement a unit-to-case serialization strategy. Although regulatory requirements for aggregation at the case level—to infer serialization of individual product units—are not due until 2023, distributors will no longer be allowed to process saleable returns unless they are serialized as of 2019. The time and effort needed to open up each and every case is, in effect, forcing the aggregation issue early. It most likely makes financial and operational sense to do this now if possible, rather than wait until 2019.
Q: What do manufacturers need to consider in order to implement an effective serialization strategy?
A: Serialization is a bigger job to manage than just putting a number on a bottle. There is masterdata and serial number management, as well as GS1 Standards and Healthcare Distribution Alliance (HDA) Guidelines to consider. Added to the mix are new software and hardware solutions being introduced to operations and IT systems, increasing the number of moving parts. Resources need to be allocated to ensure urgency and, ultimately, success. This includes a dedicated internal team, as well as external packaging solutions providers.
Q: What are some potential pitfalls to watch out for when implementing a serialization strategy?
A: Interoperability is one of the biggest pitfalls to watch. Concerning data interchange, serialization management systems must be able to communicate clearly with other systems andstakeholders along the supply chain. The same holds true internally, between line levels. An interoperable system allows for information to be seamlessly funneled along the manufacturingline as well as the supply chain.
To this end, everyone must be up-to-date on guidelines and standards. For example, Electronic Product Code Information Services (EPCIS) standards could become mandatory, but the FDA has not published any direction to adhere to a specific standard yet. It is important to stay current.
The bottom line is companies need to establish a solid chain of command to help mitigate potential problems. It’s important to ensure adequate resources, and someone high up the foodchain needs to make the choices regarding serialization strategy. This includes whether to aggregate now or hold until absolutely necessary.
Steve Peterson and Mario Simard will make their presentation, entitled “Serialization –Regulatory Compliance,” on Tuesday, Nov. 8, from 10:00 a.m. to 10:50 a.m. in the West Building, Room W-190A at McCormick Place.
The Pharma EXPO conference addresses important industry issues from production challengesto pharmaceutical packaging. Organized by the International Society for Pharmaceutical Engineering (ISPE), the program covers three topics in five sessions per day:
• Manufacturing Operations – Monday, Nov. 7
• Compliance Trends – Tuesday, Nov. 8
• Pharmaceutical Packaging – Wednesday, Nov. 9
Registration fees for the conference are $140 per session, $220 for a one-day conference pass, or $500 for a full three-day conference pass. For more info, visit www.pharmaexpo.com. Pharma EXPO is co-produced by PMMI, The Association for Packaging and Processing Technologies, and the International Society for Pharmaceutical Engineering. PharmaEXPO will be co-located with PACK EXPO International 2016. Together, the shows will feature more than 2,500 exhibitors and draw 50,000 attendees.