Management Holds the Key to Continued Process Verification
A company gets a 483 warning letter, which among other things criticizes the company’s continued process verification (CPV) approach. The observations include inadequate sampling plans, wrong statistical methods used, and company employees not understanding the statistical methods being used. Only a single quality engineer knows how the tools are being used.
So the company scurries about figuring out how to respond including the adoption of better procedures and more clearly explaining the methods being used. So what is the problem here? Who is at fault? The quality engineer? His supervisor? The plant manager? How should this situation be remedied?
The answer is: all of the above, with the major portion of responsibility on management. Juran’s 85/15 rule says that 85 percent of the problem is in the system with the remaining 15 percent due to people working in the system. Deming says that the blend is more like 94/6. The system in question is the CPV system. This entity is owned by management, who is accountable for the effectiveness of the CPV system including the design, execution and continued enhancement of the system.
Publication of the Process Validation Guidance by the U.S. Food and Drug Administration in January 2011 (FDA 2011) with its Stage 3 addressing “Continued Process Verification” has renewed Pharma and Biotech’s interest in process monitoring. Companies are working to implement guidance, and FDA is assessing progress. There has been considerable discussion regarding what is required to implement and maintain a sustainable CPV system. The FDA document, as it should, provides guidance, not a prescription for the system.
WHAT SHOULD A CPV SYSTEM LOOK LIKE?
The stated purpose of CPV is to “demonstrate that the process is in a state of control” operationally — this means that companies need to maintain process stability and capability over time — for the life of the product or process. A popular response to this need has been to install control charts and calculate capability indices. This is a step in the right direction. Control charts and process capability indices are indeed necessary, but not sufficient.
An important question is what should the CPV System look like? This, of course, depends on the organization, process and product involved. At the strategic level CPV will enable an organization to maintain process stability and capability over time enabling us to produce quality product, continually improve the process, reduce costs and remain compliant with regulations. The strategic level should also contain some goal related to maintaining competitive advantage.
At the managerial level, a procedure needs to be in place that calls for the periodic analysis, review, reporting and process improvements. Such an approach is based on the continual evaluation of process data and is done at various levels in the organization.
The operational level utilizes a variety of statistical tools such as control charts, data analytics and graphics and process variation studies to assess process performance and product quality as each batch is produced and batch-to-batch over time. Non-statistical tools such as failure modes and effects analysis are also used. These tools are linked and sequenced using documented procedures.
It has been known for ages that for a system to be effective and sustainable over time it must contain three levels of activity: Strategic, managerial and operational. A CPV approach based on only statistical tools such as control charts and process capability indices addresses only the operational level of the system. When any of the levels of activity is missing, CPV will become ineffective and fall into disrepair.
THE SECRET SAUCE – MANAGEMENT REVIEWS
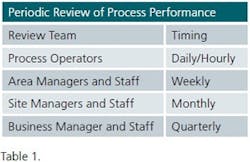
The ingredient that keeps any system operating effectively — the secret sauce — is management reviews. CPV management reviews take place at several levels in the organization at different frequencies as summarized in Table 1.
The review is not a special event. It should be an integral part of the regular management review process. A critical aspect of the reviews is the identification of the need for improvements and the assessment of recent improvement put in place. I recall a CEO admonishing his management staff to “schedule the reviews and show up. Good things will happen. In many cases you don’t have to say a word.” It is, of course, appropriate to ask questions and engage in discussion when needed.
Larry Bossidy, former COO of General Electric and chairman of Honeywell says, “Quarterly reviews help keep plans up to date and reinforce synchronization. They also give a leader a good idea about which people are on top of their businesses, which ones aren’t, and what the latter need to do” (Bossidy and Charan 2002). Management reviews work effectively to solve chronic problems. It is rare that a problem can long-term withstand regular data-based review without being solved (Snee and Hoerl 2003).
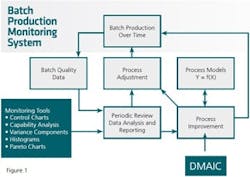
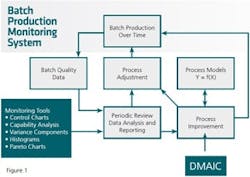
Figure 1 summarizes the critical pieces of the CPV system discussed above. Here we see process performance data regularly reviewed, resulting in process adjustments to keep the process on target and process improvements to make needed changes thereby enabling continual process improvement. This process is standard procedure, thus making the use of analytical tools an integral part of how the organization operates its processes. Further detail can be found in Snee (2010, 2011).
The improvement framework in the CPV System shown in Figure 1 is DMAIC (Define, Measure, Analyze, Improve and Control). This framework is arguably the best available framework for improvement today and is broadly used around the world (Snee and Hoerl 2003). Other improvement frameworks can be substituted should an organization so desire.
MANAGEMENT'S ROLE IN CPV
As Juran and Deming have taught us, management owns the system. Management’s responsibility regarding the system includes the following:
• Facilitation of system design and development of the strategy for how CPV will be implemented and maintained by the organization
• Continual, clear communication of the purpose and value of CPV to the organization and progress being made
• Provide resources to support the CPV, including personnel, funding, training, coaching and counseling; and
• Leading periodic reviews of CPV, which include reinforcement of desired behavior and recognition of good performance.
Through the review process, leaders learn first-hand what is going on in the operation of their processes — what is working and what isn’t working. It is also an opportunity to interact with their staffs on a real level discussing how the business is being operated rather than shaking hands during a “management tour.”
QBD CREATES AN EFFECTIVE MINDSET
A CPV system as described above links very nicely to Quality by Design (QbD). When QbD is used in product and process development, process targets are selected and it is natural to question how the process will be held on target. CPV is a natural outcome of such thinking.
Product and process understanding developed by QbD enable effective process control. The process models, Y=f(X), developed by QbD enable the effective adjustment of the process back to target when the process control procedure detects the process drifting away from target. QbD can also be used to better understand the performance of legacy processes with the resulting understanding used to produce better process control and aid in creating the associated CPV system for the legacy process.
ABOUT THE AUTHOR
Ronald D. Snee, Ph.D., is president of Snee Associates, a firm dedicated to the successful implementation of process and organizational improvement initiatives for the Pharma and Biotech Industries.
REFERENCES
1. Bossidy, L and R, Charan (2002) Execution – The Discipline of Getting Things Done, Crown Business, New York, NY
2. FDA (2011) “Guidance for Industry - Process Validation: General Principles and Practices”, US Food and Drug Administration, January 11, 2011.
3. Snee, R. D. (2010) “Critical Considerations in Monitoring Process Performance and Product Quality”, Pharmaceutical Technology – Analytical Technology and Instrumentation, October 2010, 38-42.
Snee, R. D. (2011) “Using Quality by Design to Enable CMO Manufacturing Process Development, Control and Improvement”, Pharmaceutical Outsourcing, January/February 2011, 10-18.
Snee, R. D. and R. W. Hoerl (2003) Leading Six Sigma – A Step by Step Guide Based on the Experience With General Electric and Other Six Sigma Companies, FT Prentice Hall, New York, NY.