Continuous bioprocessing technology: Adoption and future trends
Investment and adoption of continuous bioprocessing has continued to follow a steady upward trend. The trend for testing new technologies mirrors this upward trend, and in 2021 there was no indications of any slowdown in evaluation or adoption.
According to BioPlan’s 18th Annual Report of Biopharmaceutical Manufacturing, over 40% of respondents indicated ‘Upstream: Continuous processing/perfusion’ as the second-most common systems to be evaluated/tested within the next year in bioprocessing (Figure 1).
Fig 1: Novel bioprocessing systems/innovations to evaluate in next 12 months (2021)
This may not be surprising since biomass production increases can improve efficiency by increasing production of the target product, allowing for increased product recovery even in the absence of downstream process improvements. Biomanufacturers are planning to first evaluate continuous and other new bioprocessing options in-house vs. hiring CMOs to do this. This is further proven by our data in the report, showing that there are only a few CMOs being hired to evaluate new continuous bioprocess technology. Once end-user evaluations are complete and continuous bioprocessing is further adopted by manufacturers, we expect to see an uptick in CMOs being hired to implement continuous bioprocessing technologies within their facilities, for their clients.
There are several benefits to operating bioprocess continuously rather than in batch mode. These benefits include:
- Reduced costs – allows for significantly smaller-scale equipment and facilities
- Increased productivity – little need for large transfer/storage vessels upstream, with no halts between processes
- Improved quality – more controllable and less intense/stressful on the cells
- Reduced risk – greater consistency and robustness when compared with batch processing
- Increased flexibility – enables more adaptability and efficient facility utilization.
Even with upward trending interest in continuous bioprocessing technology, except for several small-scale or niche processes, upstream bioprocess steps today continue to occur in batch mode. Furthermore, continuous downstream bioprocessing is lacking as well. When implemented, it still only involves just one or a few of the many downstream unit operations. These types of technology for continuous downstream bioprocessing, such as multi-column, countercurrent, and other variations of continuous chromatography units, are just starting to enter the market.
Continuous bioprocessing operational issues
As upstream and downstream continuous bioprocessing evolve toward greater scale and robustness, batch-fed processing has continued to improve in product titer, as well as improvements in a molecule’s key quality attributes. Compared with perfusion, batch methods are still commonly viewed as simpler, cheaper, with less risk and fewer failures. ATF is becoming a relatively common method of production as related cell culture equipment improves and now includes better pumps and controls, perfusion-purposed bioreactors, perfusion-tailored media, and overall improvements in technology and operations.
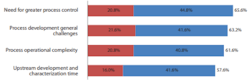
In BioPlan’s 18th Annual Report of Biopharmaceutical Manufacturing, respondents were asked what the current concerns and perceptions were when using perfusion processes (continuous bioprocessing) vs. traditional batch-fed processes in biopharmaceutical manufacturing.
'Need for greater process control' topped the list at #1 with 65.6% of respondents vs. 69% of respondents in 2020. Moving to the #2 position with 63.2% of respondents was “Process development general challenges”, and “Process operational complexity” moved to the #3 position with 61.6%, down from #1 in 2020 with 81%. (Figure 2).
Most manufacturers, including those who have adopted perfusion cell culture at small and mid-size scales, reject the technology for late-stage manufacturing. Partly to blame is the general lack of experience on the part of GMP-level staff with perfusion. Regulatory risk and concerns over scalability represent other issues.
Fig 2: Selected concerns over perfusion processes vs. batch-fed processing
Future of continuous bioprocess and process intensification
Even with concerns over scalability and regulation, the majority of users agree that both upstream and downstream continuous bioprocessing will continue to be adopted at an accelerated rate.
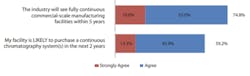
As shown in Figure 3, again, topping the list at #1, 74.8% of respondents agreed that ‘the industry will see fully continuous commercial-scale manufacturing facilities within 5-years,’ up from 65.9% in 2020. Placing in the #2 spot is a new category for the 2021 survey, ‘There are not enough large-scale single-use perfusion options (e.g., over 500L)’ with 71.6% of respondents. Remaining in the #3 position, 64.2% of respondents reported ‘perfusion systems WILL be adopted by most bioprocessing facilities.’
Fig 3: Future of continuous bioprocessing and process intensification
Perfusion: progress in adoption
Perfusion cell culture is not new, but its relatively recent role in continuous bioprocessing has been slow due to issues of scale, risk, robustness, complexity, and frankly, lack of familiarity. Fortunately, equipment and technologies supporting perfusion cell culture are also improving and making the notion of thousand-plus-liter perfusion bioreactors less risky.
Perfusion has room to grow but it will never be the solution to every biomanufacturing problem. Moreover, advances in expression systems could render productivity gains from perfusion’s higher cell densities and product titers. These improvements will be coupled with others upstream, including culture medium optimization, and improved single-use equipment. It may be true that for specific products and bioprocesses, the inclusion of perfusion or continuous chromatography will be desired, but it may well not be preferred, in deference to process simplification. Today, a new facility may only require several 500 L bioreactors operating continuously in perfusion (at 2-4g/L/day) and linked to a continuous, small, downstream process train to produce annual ton quantities of a recombinant protein. In a decade, the bioreactors used with new processes may be 5-10 times smaller, e.g., with a 50 L bioreactor matching the current output of multi-100 L or even 1,000-5,000 L conventional stirred-tank, fed-batch bioreactors.
As investment in continuous bioprocessing continues to increase, new technologies will continue to adapt and address ongoing concerns. As a result, continuous bioprocessing should continue to see increased adoption rates in the near future.