Top pharma industry predictions for 2021
In many ways, the beginning of 2021 isn’t just one of the most welcome New Years of the modern era — it could also be a turning point between the pre-COVID and post-COVID worlds.
Perhaps more than most other industries, the landscape of pharma was radically altered by the pandemic. Now, with an end to the coronavirus within reach, experts say that the industry will soon be taking stock of how it navigated the pandemic and what the sudden shifts in operations and regulations could mean in the long-term.
Here’s a look at some of the top trends experts say will impact the industry in 2021 and predictions about how the pandemic could permanently change life in pharma.
Therapeutic innovations
Biogen’s controversial Alzheimer’s treatment could become the biggest approval of the year
“At least 10 future blockbusters could be given a green light by regulators next year, and decisions do not come bigger than that pending for aducanumab, Biogen’s controversial Alzheimer’s project. For the antibody to reach the market in 2021 the FDA must rule against its advisory committee, which voted almost unanimously against approval. The agency typically sides with these panels; however, supportive FDA documents and the high-profile nature of the decision makes the outcome hard to call. Either way, the verdict is one of biopharma’s biggest 2021 events.”
There will be a huge opportunity for T-cell banking
“CAR-T cell therapies have created a huge opportunity to treat and cure certain types of cancers. But there are still a number of challenges to producing them. One of the biggest challenges is related to the quality of the raw material, which you get from the cancer patients themselves. By the time they receive CAR-T cell therapies, they are probably desperately ill and have gone through a series of treatments. So the quality of the T cells that you get is not high, or it’s difficult to get the right number of T cells. As we look ahead, I see an opportunity for T-cell collection and banking. As soon as somebody gets diagnosed with a certain cancer, the first course of treatment could be to take a T cell donation and freeze it. Then if classical therapies don’t work, they have quality T cell donations to start manufacturing CAR-T cell therapies from.”
The industry will continue to shift towards niche medicines
“Niche product indications are significantly impacting how new drugs are being developed. The way that CDMOs work with partners and the scales of drug manufacture will evolve. There is going to be a continued shift away from the large blockbuster product volumes and increasingly CDMOs will produce at smaller manufacturing scales, which will require new ways of working.”
Manufacturing capacity
Global vaccine capacity will expand
“Historically, vaccines are produced by a few companies. But given the pandemic, individual countries want a certain level of autonomy. And there are countries that are cash rich and have limited industrial diversity, such as oil-based economies, that could want to invest in new markets. That, along with the desire for autonomous control over vaccines, could lead to a production capacity expansion for vaccines in countries in Asia and the Middle East.”
“The biologics and vaccine manufacturing capacity needed globally for COVID vaccines and treatments will take a significant percentage of the overall available capacity. The growth in demand for the manufacturing capacity seen in 2020 will continue into 2021, with many companies building their own internal manufacturing capacity to meet demand.”
Small-scale manufacturing will grow
“Disruptive technologies such as cell and gene therapy will challenge the status quo and drive the industry towards rapidly deployed facilities, with smaller scale, modular and portable plants becoming increasingly utilized. This will aid the speed to market, further diversify the manufacturing footprint globally and increase the desire for real-time supply chain management and planning.”
Talent wars will continue
“The push for additional capacity in the industry has impacted the workforce and there are talent wars going on. Companies ramping up manufacturing in anticipation of new biologics and vaccines being approved to treat COVID-19 realize that this cannot be done without having the right talent in place. This has led companies to build diverse and sustainable teams.”
Supply chain
The market will return to refrigerated temps for COVID-19 vaccines
“All else equal, it is likely the market will favor vaccines that require refrigerated temperatures of 2-8 degrees Celsius. This preference for refrigerated vaccines could push pharmaceutical companies with deep frozen vaccines to determine how to maintain efficacy of the vaccine at a refrigerated temperature. If this happens, we will gain knowledge that moves pharmaceuticals’ current storage and distribution temperature from minus 80 degrees Celsius to easier-to-distribute ranges of minus 50 or minus 20 degrees Celsius, or even refrigerated temperatures.”
HPAPI capacity will grow
“An area of growth in the industry is the high-potency active pharmaceutical ingredient (HPAPI) space. The HPAPI market alone is set to grow to $33 billion by 2025, up from $16 billion in 2016. This is due to the growing importance of these ingredients in creating treatments that are not just more effective, but more patient-centric too, requiring fewer doses to achieve the same impact.
With this in mind, we can expect more contract service providers to expand their aseptic and HPAPI manufacturing capacity in order to meet higher customer demand for both.”
New API competitors will emerge
“The cell and gene therapy landscape will continue to rapidly expand and competition for raw materials will increase as programs advance into late-stage clinical trials. As we learned during the pandemic, strengthening supply chains will continue to be important. Companies will also need to think strategically about how to implement secondary sourcing plans and carry significant inventory to minimize stockout risk. This will also likely lead to the emergence of several new competitors in the vector and API supplier space.”
Novel raw materials will grow in importance
“Vaccine workflows such as mRNA and recombinant protein subunit production require novel raw materials in manufacturing and formulation steps alongside step change improvements in scale, documentation and characterization of impurities. At the same time, we must not forget that raw material inputs and single-use components for vaccine production are often shared with mAbs, where operational requirements are already significant and time sensitive.
Balancing the increased production of vaccines with these other therapies will require innovative solutions in order to meet the needs of all patient populations.”
Investments in reshoring will increase
“Pharma had recently been reversing the trend from having a global supply network to buying more and holding onto more emergency supply for resiliency. But that will simply create inventory problems. So pharma companies are now thinking about how they can bring manufacturing onshore in a tax-efficient fashion and then automate the process to lower costs.
EY recently surveyed 500 CEOs of $1B+ companies to understand post-election priorities; 64 percent said they would acquire or build more domestic production, given the anticipated onshoring incentives with continued ‘Buy American’ theme. The industry is not chasing low-cost labor arbitrage anymore, because then you are exposing yourself to black swan events like the pandemic. So reshoring is expected to get more traction.”
Pharmacy Benefit Management (PBM) companies will push against reshoring
“The U.S. is at a strategic disadvantage when it comes to drug supplies and they can be used as a weapon against the U.S. population. Viable strategies are needed to bring generic drug manufacturing home. The U.S. government has talked about this, but is inept to have a concrete plan. PBMs will make sure drug manufacturing does not come to the U.S. as their profitability will be lowered. This would not be acceptable as PBMs will have to raise generic drug prices to retain their profits.”
Manufacturing technologies
Equipment will be more integrated
“2021 will continue the renewed focus on operational efficiency across equipment assets. For tablet compression equipment, this can mean additional turrets to maximize output and streamline changeover — or the implementation of on-line tablet testing systems to permit a single operator to monitor the multiple machines. The tablet press control system will be increasingly integrated to the central plant system to facilitate domain login, central product recipe management, and central batch data storage.”
COVID will trigger many other small — but critical — innovations
“COVID-19 vaccines have created a heightened focus on what will become the most widespread cold-chain inoculation effort in world history. This will inevitably lead to a cascading subset of obstacles that suppliers will need to address based on their own specific niches.
For example, one of the 'micro-factors' that come into play with vaccines that have to be stored at ultra-cold temperatures is that adhesives — such as the glue that holds together many secondary packaging folding cartons — can't withstand that level of deep freeze; the glue crystalizes and expands, and the carton joint can pop apart. This has led to a situation where something as simple as paperboard has become a mission-critical packaging platform. And in this case, it has led to innovation in devising glue-free carton options.”
The ultra-low temperature (ULT) infrastructure rapidly put in place will help make precision medicine mainstream
“The infrastructure built now to protect the COVID-19 vaccine supply chain will have long-term benefit when it comes to the accessibility of precision medicine as more of these treatments are brought to market. As an added benefit, the range of temperature capability enabled by ULT infrastructure will shorten, if not eliminate, the need for stability testing of many kinds of drugs, which typically takes months. Dry ice will continue to play a role in larger-scale distribution where providers trained and equipped to handle this volatile substance carry product for a short time before handing off to the next. However, for last mile delivery and long-term storage in health facilities and pharmacies, ULT freezers will have greater long-term value and better manageability while skirting the liability and safety issues associated with dry ice.”
Pharma companies will build a stronger quality culture
“COVID-19 had companies shifting to innovation mode to develop therapies, tests and vaccines to combat the pandemic. And they needed to move quickly.
On top of that now is the FDA’s announcement of a Quality Management Maturity (QMM) pilot program. The purpose of the program is to gain insight from third-party assessments of a manufacturer’s quality management system to inform a future development of an FDA rating system to characterize a manufacturer’s QMM. As stated in the announcement, ‘for manufacturers with a mature quality management system, the FDA can exercise a more flexible regulatory approach. Leading toward the goal of producing high-quality drug products without extensive regulatory oversight.’
While these are very different events, the common thread is digitally integrated quality and manufacturing processes, because that is what allows a manufacturer to deliver products quickly while maintaining the highest levels of quality.”
Drug development
R&D for personalized medicines will continue
“Projections for the industry are forecasting increased investment into R&D focused on the development of methods for early disease detection, development of ‘personalized medicine’ to match patients with customized drugs, and the development of more precise and targeted drug therapies based upon greater understanding of the mechanisms of various disease states.”
The market for sterile injectables will expand
“There’s an increasing need for innovative products to treat the rising cases of chronic diseases and many will be delivered through prefilled syringes. This is linked to the broader trends over the past decade towards home healthcare and self-administration of sterile injectables. The global prefilled syringes market size is projected to reach $9.53 billion by 2026, exhibiting a CAGR of 10.4 percent.”
R&D will focus on immunity
“The pandemic has slowed down R&D in many areas outside of vaccines, including oncology, although this is picking up again. This has led to a lot of uncertainty, and while oncology will still be the leading R&D area in terms of investment, we're starting to see a real focus on immunity. One of the things that makes cancer so tough to treat is that it comes from your own body. This makes it difficult to target without killing other cells.”
Infectious disease investments will increase
“To date, oncology indications have been the most attractive target for capital investment in both the pharma and biotech sectors. While many new modalities, ranging from CRISPR to mRNA and cell therapy have gained momentum, they are predominantly aimed at various liquid and solid tumors. In the past, infectious disease, irrespective of COVID, received a comparatively marginal amount of investment despite having a greater impact on overall global health. The new found value in Gilead’s repurposed remdesevir, developed for Ebola but finding success in COVID, may motivate pharma and investors alike to allocate greater resources to currently lower value, but greater impact, infectious disease indications beyond coronaviruses.”
COVID-19 will strain other clinical trials
“Due to COVID, many clinical trials will continue to face challenges with enrolling patients. This will negatively impact the number of trials that remain operational and limit the number of new programs in 2021.”
Regulatory changes
The industry will push to keep new efficiencies
“During the coronavirus there was a huge amount of activity and a lot of regulatory barriers that slowed things down were set aside. Almost immediately you started hearing from industry that maybe we could do this going forward.
One potential area for reform is post-approval changes. One of the big problems with COVID is that you have outbreaks happening in waves and degrading manufacturing capabilities at different facilities at different points in time. So many companies have had to request approval to switch manufacturing to another location. That process can be very involved — but it had to be done very quickly during the coronavirus. So there’s going to be a look at with these expedited procedures and many will ask: Maybe it didn’t get the scrutiny they normally would, but if everything worked out well why don’t we do that going forward?”
Regulators will leverage digital tools
“Regulators will continue leveraging new and existing remote approaches, from records-review to product-sampling and quality testing. International collaboration among regulators will continue to improve as well, especially under the incoming Biden administration. That means you should plan for the inevitable fact that any inspectors you work with, regardless of the regulatory body they represent, will also be sharing the information they collect with their counterparts around the world.”
There will be major changes to EMA Annex 1
“We will see a radically different EMA Annex 1: Manufacture of Sterile Products document that more fully reflects the diverse reality of contemporary sterile product manufacturing across the globe. While the European Medicines Agency (EMA) may have thought the draft was non-controversial, significant concerns have been raised by major manufacturers and others including:
- The document is rooted in technologies used in the late 1970's and this limits its utility in today's more automated processing environments;
- An over-emphasis on sampling and testing to confirm process/product suitability, which is antithetical to the widespread use of validation;
- A general denigration in the draft of the most capable aseptic processing technologies by essentially equating their performance to manned cleanrooms;
- A continued belief in the reliability of environmental monitoring, sterility testing and process simulation as definitive evidence of asepsis;
- Inadequate consideration of global regulations and compendia that describe a very different form of control.
The extensive comments submitted on the second draft suggests that a substantial difference in perspective remains and that further changes will be forthcoming to develop a document more suited to the current environment.”
Market trends
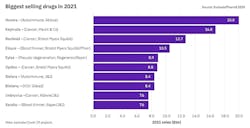
Humira will keep its top selling spot — for now
“Oncology will continue to be a major driver of the sector’s topline growth in 2021. Six of the 10 biggest new sales generators are treatments for various cancers, and three of these are anti-PD(L1) antibodies. Keytruda is king here — the Merck & Co. product is forecast to become the world’s biggest-selling drug in 2023, wresting the crown from Abbvie’s Humira.”
M&A will bounce back
“In 2020, M&A was down in pharma — about 40 percent below 2019. There was an uncertain environment, and not enough de-risked assets. Now, executives are seeing a light at the end of the tunnel with vaccines getting broad distribution and legislative stimulus coming. Pharma is also targeting more difficult therapeutic areas like CNS diseases and Alzheimer’s, so they have to rely on external innovation.
2019 had an all-time high of $378 billion in M&A in pharma, but over the last 10 years, the average has been about $200 billion a year. In 2021, we expect M&A to return to at least the $200 billion level.”
Doctored or fake vaccination cards will be circulated
“Operation Warp Speed will include COVID-19 Vaccination Record Cards with each vaccination kit that include personally identifiable information (PII) such as full name, date of birth, patient ID number, and pertinent COVID-19 vaccine information like the vaccine manufacturer, dosage dates, and where the vaccine was administered. There is still no global consensus on which activities will require a COVID-19 immunization record, though signs seem to point to some forms of travel and returning to work, as early frontiers. This will undoubtedly create a market for fraudulent vaccination records. The proliferation of high-quality photos of CDC COVID-19 vaccination records on social media platforms, including PII, QR codes, hospital information, and health care provider signatures, make a threat actor’s job that much easier. The only remaining question is how big the fake COVID-19 immunization card market will be.”
The biopharma revolution will take hold
“The development of COVID-19 vaccines is just the most compelling example of the potential of what MGI calls the ‘Bio Revolution’ — biomolecules, biosystems, biomachines, and biocomputing. In a report published in May 2020, MGI estimated that ‘45 percent of the global disease burden could be addressed with capabilities that are scientifically conceivable today.’ For example, gene-editing technologies could curb malaria, which kills more than 250,000 people a year. Cellular therapies could repair or even replace damaged cells and tissues. New kinds of vaccines could be applied to noncommunicable diseases, including cancer and heart disease.
The potential of the Bio Revolution goes well beyond health; as much as 60 percent of the physical inputs to the global economy, according to MGI, could theoretically be produced biologically. Examples include agriculture (genetic modification to create heat- or drought-resistant crops or to address conditions such as vitamin-A deficiency), energy (genetically engineered microbes to create biofuels), and materials (artificial spider silk and self-repairing fabrics). Those and other applications feasible through current technology could create trillions of dollars in economic impact over the next decade.”