Postponement is a supply chain strategy for rapid response to changing market conditions. Lead times are reduced, working capital is cut and waste is minimized. In contrast to traditional make-to-forecast approaches, postponement is a make-to-order tactic, where products are rapidly customized from stocks of almost complete products, often in close proximity to customers.
Well-known businesses such as Dell use postponement to keep remarkably low inventories while maintaining short lead times. Toyota also employs postponement strategies to make vehicles to specific customer requirements, without excess inventory or long lead times.
There is a growing need for postponement in pharmaceutical manufacturing. COVID-19 increased the demand for patient-centric supply chains and approvals for high-value, small-patient population medicines are rising. Meanwhile, manufacturers continually need to cut costs and release working capital.
However, despite being used successfully in other industries, postponement’s use in pharma manufacturing has been somewhat rare due to complexity and limited awareness.
A solution for pharma
While postponement is a straightforward concept, it is difficult to design and apply. Typically, pharma executives want to know:
- Why postponement is relevant, and what benefits will it deliver to my business?
- Where should postponement occur? Central location or in key markets?
- Where should product manufacturing processes be decoupled for the greatest flexibility?
- Should postponement be developed in-house or purchased as a service?
- How can products be simplified to support postponement strategies?
- Who are the best partners to operate the new model?
- How does existing operational performance management need to be adapted?
- Do sales and operations planning governance and processes need to change or do companies need to adopt other approaches like demand-driven material requirements planning?
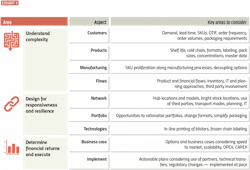
To answer these questions, leaders must understand the inherent complexity in employing postponement strategies, accounting for multiple factors, including customers, products, manufacturing, and product and financial flows. It’s also crucial to have a firm grasp on the best ways to build responsiveness and resilience into networks, as well as the financial case for making the change. This requires cross-functional engagement, a structured analysis and a robust approach, set out in Exhibit 1.
Pharma’s postponement model
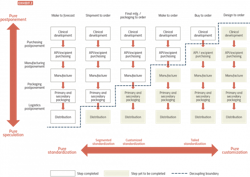
In a postponement model, products are held in semi-finished states and finalized (often labeling and packaging) based on market demand, such as customer orders or newly published regulations. Postponement enables manufacturers to supply their products to markets in response to known demand as opposed to forecasted demand.
Lean production methods, including rapid quality checks and flexible materials management, are needed support the final stages of postponement. This make-to-order configuration model also requires robust and nimble IT systems.
Several logistics providers can perform postponement, or manufacturers can do it themselves. The choice depends on complexity, capabilities and risk appetite.
Understand rationale and complexity
The rationale for postponement needs to be clear from the outset. Analysis should show how issues would be resolved through its application. For instance, companies can avoid running out of stock by simply holding more inventory; however, that approach increases working capital, heightens the risk of products expiring and leads to more waste and obsolescence.
Postponement can address these potential challenges, but leaders must first focus on understanding the requirements of their customers. What are their expected lead times, service levels and order frequency? That will determine how the network is set up. For instance, can customers be supplied from a central global postponement hub, or are regional or country configurations needed?
Secondly, product characteristics such as shelf life, temperature requirements, formats and pack sizes need to be analyzed and commonalities identified. Groupings of similar products
can help to optimize utilization of assets within a postponement setup.
And finally, leaders should map out manufacturing steps to understand where SKUs proliferate. For example, products might be the same until the blistering stage in packaging, after which many country-specific SKUs are generated as blisters are printed and packaging occurs.
A key strategy in dealing with these complexities is to develop a “decoupling point” where the process is disconnected and a controlled stock of partially finished products is established. From this point, products are rapidly customized to customers’ orders.
Leaders should map out the product, informational and financial flows to understand the issues and how the many stakeholders along the supply chain are involved. Additionally, the change from a make-to-forecast to a make-to-order process means management must understand how its existing planning system operates.
Exhibit 2 summarizes these considerations and can be used as a starting point to think through network design. For instance, a network decoupled at an early manufacturing stage would look
quite different to one with a single postponement hub for secondary packaging.
Design for responsiveness and resilience
To design a postponement supply chain network, organizations should be guided by important factors, such as OTIF, lead time, working capital, CAPEX or OPEX. Scenarios can be developed and assessed to determine locations of hubs and bright stocks (unlabeled finished products) locations.
The right level of automation for postponement hubs also needs to be determined. What portion of the network should be manual, semi-automatic or fully automatic? In addition, any investments required to build out the network should be included in the business case for the project.
Organizations have several options for tapping into third-party expertise and the efficiencies they can provide. Contract management organizations and logistics service providers offer specialist capabilities to help companies gain a deeper understanding of appropriate modes of transportation, cost per product, lead time and any working capital implications.
In parallel to defining the big picture, leaders must pay close attention to such considerations as master data and how the organization’s enterprise resource management and information technology services would need to be changed to capture the various categories of distribution in the new network. Different order points may be needed to differentiate between finished products and bright stock, for instance.
To increase flexibility, it is important to simplify packaging, including standardizing text, symbols and languages. These changes require significant regulatory input, particularly around new material numbers and re-registrations. All these issues need to be facilitated through any project.
As new lines are set up, some will need to be decoupled, or require moving production equipment from one location to another. At each step, the necessary quality approvals will need to be obtained.
Whichever route companies decide on, master and transaction data must be 100% accurate and supported by robust master data processes and IT systems.
Determine financial returns and execute
Companies can benefit by evaluating two to three network options in terms of OPEX and CAPEX, as well as key criteria such as speed to market, scalability and ease
of implementation.
Once a suitable option is agreed upon, leaders can develop an implementation plan that takes into consideration the use of third parties, technology testing and purchasing, technical transfers, data improvement and regulatory changes, among other factors. They should also allocate dedicated project resources to implement the changes, including use of project management organization and tracking tools to measure progress and ensure milestones are achieved as intended.
More responsive and resilient supply chains are needed in pharma manufacturing, and postponement is an effective way to accomplish this. We hope this overview will inspire you to consider implementing this supply chain method to achieve the next level of performance within your pharma organization.