Outsourcing in biomanufacturing has long been on a steady upward trend. This year, there are few indications of significant slowdown. This is partly due to COVID-19 — according to our recent research, 70% of the biopharma industry is planning to increase their future outsourcing in an effort to address supply chain risks.
Small companies continue to be major sources for innovation, and many have an active pipeline of products in development. Since many smaller companies have no manufacturing capabilities, and often don’t plan to develop those core competencies, these companies need the technical expertise of contract manufacturing organizations (CMOs) to help bring their products to clinical stages, and eventually to commercial manufacturing operations. Larger, more established companies will also continue to search for partners to get their established products outsourced in order to free up internal capacity for new, upcoming products in their pipelines. This trend is not all that surprising.
But what is surprising from our Annual Report of Biopharmaceutical Manufacturing is the growth in international outsourcing, or offshoring. Our survey found that nearly two-thirds of respondents indicate they expect to increase their levels of offshoring in process development.
Increase in offshoring of biologics
In the 2021 Annual Report, we evaluated responses over 10 years (from 2011 to 2021) regarding the percentage of biomanufacturers/developers that plan to offshore (outsource internationally) at least some (any) of the indicated activities within the next five years.
While all areas of manufacturing reported record or near-record levels of outsourced and expected outsourced activities, in 2021 alone, there was a significant increase in planned offshoring for all areas. An example of this can be seen in process development specifically, with 64.4% of developer respondents reported expected offshoring, up significantly from 34.9% in 2020, and the second-highest recorded amount in this area since collecting data (2019 levels were 69.1%).
“Biomanufacturing operations” is another example where respondents report a significant increase in expected offshoring in the next five years, with 69.3% of respondents, up significantly from 34.9% in 2020, and the highest recorded amount in this area since collecting data. All other areas tracked for offshoring trends indicated record levels of expected offshoring. Offshoring continues to be an attractive option for companies looking to expand their reach outside their domestic markets, for those seeking second source manufacturing processes, and for other reasons.
Offshoring is still perceived to provide cost savings. However, we found that other benefits, traditionally not associated with international CMOs, are also important to those looking to offshore their manufacturing. These features can be harder to quantify than calculated cost savings. Factors such as quality and reliability, as well as regulatory competence, are increasingly being associated with offshoring among emerging market CMOs as well.
These factors are recognized as critical for decision-makers to consider when choosing an international partner. In a 2020 survey, we asked respondents: “Which of the following factors would you consider MOST IMPORTANT if outsourcing to new regions, such as China, India, and Brazil?” We found that factors associated with quality and reliability were on the top of the list. The top five selected leading factors were:
- Meets international GMP standards,67.2% of respondents
- Protect intellectual property, 52.5% of respondents
- Comply with my company’s quality standards, 50.8% of respondents
- Pricing/cost of services, 37.7% of respondents
- Demonstrated ability to do the work, 34.4% of respondents
Based on these results, it is clear that respondents that are offshoring expect similar levels of demonstrated quality that are in line with GMP standards — and this is significantly more critical than any potential cost savings.
Favorite offshoring destinations
Regulatory concerns in outsourcing continue to be on the minds of biomanufacturers today.
The necessary expertise primarily exists in more established markets. A lack of regulatory and GMP manufacturing expertise required by decision-makers in developed countries can create considerable hurdles for developing market CMOs.
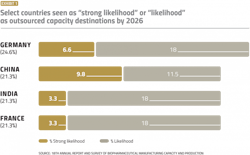
Perceived lack of regulatory and GMP manufacturing expertise trickles down to the decision-making process when choosing which country to favor when offshoring manufacturing. In our annual report, we evaluated the “strong likelihood” or “likelihood” of U.S. respondents considering countries for international outsourcing. We identified 18 major countries that may be likely candidates for international outsourcing. For U.S.-based companies, the top four results can be seen in Exhibit 1.
In 2021, Germany leads the group with 24.6% and China comes second with 21.3% of respondents indicating them as at least likely candidates for offshoring. For China, this differs sharply from the situation eight years ago when the country barely registered as a possible outsourcing destination.
For European decision-makers, the U.S. and Germany tie for first, with 42.9% of respondents indicating the countries would be a “likely” or “strong likely” international outsourcing destination.
So far, most outsourcing to CMOs in developing countries has involved less-regulated biosimilar and/or biogeneric drug manufacturing. These types of services have typified offshoring from developed to developing countries so far. While increasing overall, it can be seen that at least for the near future, offshoring of manufacturing tasks to CMOs in other countries, particularly to developing countries, will continue to be selective and not a major threat to the U.S. and European CMOs for other areas of the market. This could change as these developing countries invest in capabilities to accommodate the regulatory requirements in larger markets, making them more attractive destinations for some of the more critical and more regulated manufacturing projects.
However, China in particular with its ongoing investments from Western CMOs and its emerging domestic industry will likely become a global player in the CMO space for biologics within five to 10 years and potentially emerge as a top choice for offshoring regulated and less-regulated manufacturing.
Offshoring to help gain a foothold in China’s developing market
One often overlooked benefit of offshoring is that it allows developers to quickly gain a visible foothold in a developing market. An example of this can be seen in China. BioPlan recently published an extensive study and directory of CMOs in China (PRC) As a result of an increase in growth of its domestic biopharmaceutical industry and bioprocessing activities, China’s CMOs have also rapidly expanded their capacity to meet demand in China and have become increasingly attractive options for Western companies looking to offshore. This increase has occurred to meet demand from the large domestic population, particularly in terms of the expansion of biogeneric monoclonal antibodies manufacturing for domestic consumption.
While a large part of this increase can be credited to the growing number of biopharmaceutical developers and the potential of the domestic market in China, this expansion has also occurred due to outsourcing from Western companies. CMOs currently play a big role in how Western biopharmaceutical developers will gain a foothold in China, utilizing their production capabilities to meet the domestic demand in the area. This has been spurred by changing central government laws and regulations that traditionally ruled out drugs being manufactured by CMOs and other third parties. This interest among Western companies in outsourcing bioprocessing to China CMOs has been increasing over the decade. China was cited by 40% of respondents as an outsourcing destination, compared with only 2.8% back in 2009.
Regulatory concerns for CMOs outside the U.S. and EU
The issue of regulatory competence is especially a concern when developers consider outsourcing of core activities for which regulatory requirements related to manufacturing are more stringent, such as GMP manufacturing.
Regulatory approvals for marketing products for the U.S. and EU markets are increasing for other developed and developing countries. However, as it currently stands, most rest-of-world facilities have only approvals or inspections related to making products for local and regional markets, or simply operate with no regulatory approvals or inspections. As a result, many of the activities offshored to these emerging countries are not for GMP operations.
However, this is changing. For example, biosimilar products made in India and China will increasingly become available in major markets, and several Chinese companies are launching biosimilars in the U.S. and EU markets. At the same time, as other countries increase their regulatory approvals, they will increasingly become candidates for offshoring opportunities as well. Eventually, the current leading developing Asian countries with CMOs now considered and used by Western customers will likely see much of their business shift to new CMOs in even lower-cost countries.
As the markets for developing countries continue to evolve, offshoring trends will continue to increase to help Western companies meet the demand represented by these emerging markets. We anticipate that the trends mentioned here will continue for five to 10 years, with the potential for continuity past that.