The parallel path
Recent events have shown that unexpected medical needs can result in stock sector volatility, instability in multiple areas of the economy from large to small businesses, strict border controls and limits on free movement. These conditions pose challenges to the pharmaceutical sector, highlighting the need to rethink medical device, testing and drug supply chains as well as the speed at which solutions are delivered to society.
Pharma manufacturers leading the development of drug and vaccine products realize that new technologies are the strategic drivers of innovation, safety and speed. As clinical trials are finalized, the ability to rapidly pivot to produce new products and ramp-up to meet production demand severely tests technology infrastructures.
The ability to simulate, visualize and qualify manufacturing strategies — even in advance of the typical commercial readiness timelines — are the levers needed to speed execution. The availability of real-time manufacturing data, with enhanced visibility and the power of predictive insights, will enable agile response to demand fluctuations.
A fast-track solution
Some believe the solution to most efficiently ramp up production of potential clinical therapies is to facilitate development of full commercial scale manufacturing earlier, while treatments and preventions are still in clinical trials.
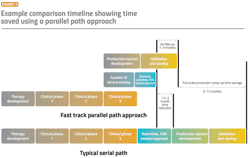
A pre-structured process automation solution can be available for use in development applications in months and enable pharma companies to immediately scale up to full production once regulatory approval is granted. Parallel path development of an engineered solution can start with minimum formulation details on a final therapy.
The manufacturer can even start to apply digital transformation to the manual steps during clinical trials to better consolidate and analyze data and prepare electronic submittals for regulatory review. The project can start with a supervisory control and data acquisition (SCADA) system overlaying the manual data collection. Then, data can be used to prepare for the final production automation design. The best SCADA solution to use is one that can be easily transformed into a full-blown distributed control system (DCS) solution when needed, as production becomes more complex.
Fast-track automation includes process automation elements that can be rapidly configured virtually and remotely once a therapy is approved and ready to be produced for commercial distribution. A treatment or vaccine can go from approval to production in as little as four weeks depending on process requirements (Exhibit 1).
Deliverables and implementation
A delivered fast track automation solution may include an automation system, SCADA or DCS depending on process requirements, detailed engineering of the system design and applications, project execution and validation, and process equipment skids specified and procured by an authorized solution partner.
The complete system is scalable to meet the needs of varying process requirements and integration is already pre-planned. Automation can be planned for processing several potential therapies until final trial results and approvals are complete. That way, a pre-prepared manufacturing system is ready to go for whichever therapy is approved.
A project delivery “ecosystem” can be positioned to enable rapid delivery of both appropriate expertise and necessary equipment. Global centers of excellence, engineering teams, internal project execution and authorized solution providers can form a team to provide expert installation. This project ecosystem can serve the needs of pharma at an expedited pace.
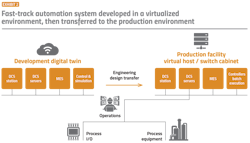
Implementation starts with development of the automation system on a virtual engineering platform in a private cloud. The design and engineering may include application configuration and qualification services provided by a selected authorized channel solution provider. If a manufacturer wants to repurpose existing process equipment, the integrator will work with the facility to integrate the identified equipment into the final design.
Hardware is then assembled and shipped to the data center, where the facility’s servers, virtual hosts and switches are set up; and to the site, where the process equipment, skids and controllers are installed. Finally, the engineering design is transferred from the virtual host to the production system.
Technologies enabling faster startup
A manufacturer should choose an automation vendor that has continued to evolve and innovate, generating significant advances in automation technology. Integrated, yet “open” technology promotes the ability to integrate seamlessly with most modern equipment from any manufacturer and should leverage a breadth of complete virtual and cloud solutions. Some examples of automation advances include:
Lean automation project execution: A lean execution solution enhances project implementation services with standardized equipment cabinets, virtualization, cloud engineering and auto-device commissioning for the greatest efficiency and flexibility. Lean implementation enables engineering to be done from anywhere in the world. The advantage of lean project implementation comes from the use of standardized builds, parallel path development and automated engineering transfer, so that configuration is automatic, remote and there is no waiting on last-minute design changes. Employing lean automation project execution alone can enable a large capital project to potentially realize substantial capital savings in automation infrastructure and decrease in time to startup.
Reduced validation effort: An easy transition from recipe testing to execution reduces qualification efforts. Configurations tested in a virtualized development environment (as a development “digital twin”) can be downloaded to the production environment without change, moving control strategies to production effortlessly, without modification or reassignment (Exhibit 2).
Batch in the controller: Batch executed in the process controller reduces the engineering effort to design, modify and configure a batch system without shutting down, and reduces recipe maintenance through equipment independent master recipes. It helps manufacturers produce an increasing number of different products cost-effectively and rapidly. The solution should be aligned with international batch standards and include the full ISA S88 batch standard model in the controller.
Remote control from a data center: Using the power of the Industrial Internet of Things (IIoT), an automation vendor can consolidate data and move control to a centralized local or remote data center in a private cloud. The resulting “digital twin” of the control process allows for testing of new recipes and qualification of changes to the automation system offline. The enabler is batch execution located in the controller.
Flexible input/output: By decoupling the data input/output units (I/O) from a specified controller using a highly integrated virtualized environment, the I/O for each piece of process equipment can be connected to virtual data center controllers. This allows personnel to remotely change the production process online. The control system can assign controller power to any I/O as needed (Exhibit 3). This reduces the number of I/O units needed and makes the system simpler, more efficient and flexible.
This smart assignment of control compute can be used in addition to the convenience of universal I/O modules, which can be changed to any digital or analog configuration remotely and instantly without changing out or adding additional equipment when changing automation strategy. This allows late binding of automation strategy at the I/O and equipment level.
Modern benefits
Modern technology components and project execution come together to provide the flexibility necessary for faster advancement from clinical trials to full-scale production of new treatments.
Working together with modular facility construction, manufacturers can quickly go from trial and approval to production. There is no waiting on design and engineering. A pre-designed automation process enables parallel work to start immediately, so manufacturers are ready to go once a therapy is approved.
Pharma manufacturers are finding it easier to move their digital transformation into the future with technologies enabling flexible control, virtual engineering and simulation and real-time data and operational visualization. These enablers provide the agility and speed-to-market for better delivery of medical solutions to those in need.