The pharma industry was already in the midst of a slow but steady adoption of digital technologies as a way of beefing up operational capabilities. Once COVID-19 hit, and the industry was asked to suddenly quicken its pace in every way, the need for digital tools increased even more.
“Pharma supply chains are digitizing across the world, involving many smart advancements like big data analytics, AI and large-scale automation that break down silos and improve efficiency,” says Lee Patty, vice president and general manager at NiceLabel Americas. “And with the current pandemic, this trend has only accelerated.”
Whether it’s through increasing connectivity throughout all areas of operations, providing new tools for analyzing data or helping pharma overcome barriers to advanced technologies, vendors are innovating IIoT-based solutions that make the digital transformation more seamless than ever before.
Centralized labeling
Small mistakes in labeling can lead to major disruptions in manufacturing.
“[Labeling problems] not only can lead to dangerous errors and recalls, but they can also halt digital transformations, reduce traceability, hinder processes like serialization and aggregation and even stall speed-to-market of essential goods,” Patty explains.
Despite the industry’s focus on adopting digital tools, Patty says that companies often overlook the value of a central labeling solution — but this is where NiceLabel’s Label Cloud comes in.
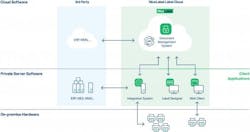
Label Cloud was developed to allow businesses of all sizes and industries to digitally transform their factory and warehouse labeling processes at a rapid pace. NiceLabel says that the solution integrates easily with other business systems such as ERP and MES, does not require additional IT investment to adopt and is particularly suited for pharma, where regulations change frequently, regional standards differ widely, and consumer safety is of the utmost importance.
“It can be deployed in hours, and with it, users can centrally manage label design, product data and quality control from the cloud and print their own labels locally, from any printer type or alongside any business system,” Patty says.
NiceLabel’s Label Cloud automates quality assurance to eliminate manual quality control processes.
Streamlined analytics
With the push to get medicines to market quickly, increased digitization gives companies the flexibility they need to address this issue by handling specialized products and shorter production runs.
“As pharma companies drive toward implementing these changes, establishing unparalleled operational efficiency and agility is critical,” Lisa J. Graham, the vice president of Analytics Engineering, Seeq Corporation, explains. According to Graham, Seeq’s advanced analytics software can underpin the “new processes, workflows and culture shifts required for success.”
To help streamline connectivity throughout all phases of drug development, Seeq recently launched a beta version of Data Lab, which works alongside two other Seeq applications — Workbench and Organizer. Data Lab was developed to help data scientists expand their Seeq analytics efforts to the rich ecosystem of Python libraries and for direct participation in process data analytics.
“Data Lab also enables data scientists to find insights using machine learning algorithms and libraries, system integrators to create custom analytics for their clients, and asset vendors to enrich their remote monitoring and predictive analytics services for their customers,” the company says.
The three applications connect to a Seeq server to facilitate collaboration, access connected data sources, and enable administrative control.
Overcoming implementation barriers
Despite the promise of digital solutions such as AI, pharma companies are often still reluctant to make the switch.
“AI holds a strategic, transformative business value,” says Massimo Bonardi, managing and technical director for Antares Vision. “However, limited availability of high-quality data, a lack of processes for collection of knowledge, insufficient understanding of risks associated with the adoption of AI, company legacy culture and regulation can all act as barriers to widespread adoption.”
According to Bonardi, Antares’ AVionics solution can help users overcome these challenges.
Originally developed for OEE performance tracking, Antares says the AVionics software solutions “can now be utilized to track overall manufacturing efficiencies, ensure process quality, predict equipment maintenance and detail sustainability parameters.”
While tracking various key performance indicators (KPIs), AVionics streamlines data into a dashboard that gives operators information from production and packaging lines that can then be used to improve business processes at all levels — from line-level manufacturing through overarching, enterprise-level metrics development.
“Powered by AI, the data collected by AVionics can be used to solve specific problems like anomaly detection, to predict potential production issues, and to enable a fully managed AI governance,” Bonardi says.
Data transparency
Although industrial monitoring devices are now widespread throughout manufacturing, solutions that provide real-time status updates are just now hitting the scene. According to Bethany Silva, industry manager for Life Sciences, Endress+Hauser USA, the ability to have real-time data and transparency is enabling a higher level of predictability in pharma.
“Pharmaceutical manufacturers are seeking ways to integrate data throughout their entire ecosystem in a manner fully transparent to all the players involved, from the suppliers of raw materials, components, and parts, to the transporters of those supplies,” Silva says.
To that end, Endress+Hauser recently launched its Micropilot FWR30, the company’s first cloud-connected radar level instrument, which provides full transparency in the storage and transport of liquids. As the world’s first 80 GHz wireless IIoT instrument, it continuously records measurement data that can be accessed at any time thanks to the device’s cloud connection.
The Micropilot FWR30 can be installed in less than three minutes and can then be used for level measurement, tracking and inventory management of mobile and stationary plastic tanks.
“Connected devices, such as the Micropilot FWR30, can easily track the availability of materials in real-time, allowing for better and more efficient inventory control and reduced costs,” Silva says.
Top image: Through AI algorithms based on machine-learning processes, Antares' AVionics is designed to further enhance system accuracy and reliability over time.