Modernizing the links in a global supply chain
Disruptions in today’s global supply chains are numerous, often occurring unexpectedly with significant impact. Unforeseen global events, such as the current coronavirus, can have a significant impact on pharmaceutical supply chains. To mitigate the risk of such events impacting manufacturing operations, drugmakers have extended their supply chains and diversified their network of suppliers globally. In fact, nearly 88 percent of active pharmaceutical ingredients (APIs) for drugs that are sold in the U.S. are not made in the U.S.
Europe is the largest manufacturer of APIs, with 26 percent of the market share, followed by India (18 percent) and China (13 percent) — though the World Health Organization says China’s market share is much higher. As suppliers become more global, manufacturers must learn to work with them as an extension of their own operations. However, it is becoming difficult to manage an increasingly complex supplier ecosystem as large suppliers establish new manufacturing sites to increase capacity and smaller suppliers are acquired or divested.
Adding to the complexity, cell and gene therapy innovations require extensive reliance on specialized suppliers which limits sourcing options. If any one supplier suddenly closes, encounters manufacturing snags, decreases capacity, is impacted by a natural disaster, or detects batch contaminants, drugmakers could be left without a reliable substitute. For example, unacceptable levels of N-Nitrosodimethylamine (NDMA), a probable carcinogen, from a Chinese API supplier triggered product recalls in 2018 and in 2020.
Modern, cloud-based systems improve transparency so pharma companies can better collaborate with suppliers and partners, including contract manufacturing organizations (CMOs), to mitigate quality risks. Unfortunately, many companies still use manual processes and a fragmented landscape of legacy systems that make it difficult to manage today’s longer, more complicaed supply chains. These challenges have taken a major toll on the industry with a surge in product shortages, recalls and regulatory citations.
Non-routine quality events — such as major observations, recalls, warning letters, and consent decrees, along with associated warranties and lawsuits — cost the industry between $2.5 and $5 billion per year on average. And, these figures do not include losses of goodwill and market share. Further, major quality events can also have a long-term impact on a company’s reputation. In the past decade, an average of one company per year has seen a 10 percent drop in share price after a single major product recall.
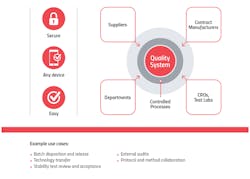
A unified quality cloud enables seamless connections and collaboration between internal and external stakeholders, including suppliers and CMOs, for better visibility from end-to-end.
Here are three ways advanced quality management systems improve supply chain management and safeguard patients:
1. Digitize distribution and management of supplier process controls
Pharma manufacturers create, distribute, update, and supply all partners with manuals that establish detailed policies, guidelines, and standards that outline specifically how manufacturing processes are to take place at their facilities. Manuals include detailed documentation on approved specifications, master batch records, test methods, and other workflows to ensure compliance with the manufacturer’s guidelines and regulatory standards.
Pharma companies initiate needed updates, such as incorporating new specifications, to these manuals and send to their suppliers and partners. With a cloud-based quality management system, pharmaceutical companies are automatically alerted when these changes have been received, viewed and implemented. This provides companies with greater visibility into and oversight of partner operations. Creating, revising and distributing these control processes to third parties is now an easy and secure process, because it can be implemented through the system.
The quality management system also allows companies to manage each supplier’s access privileges based on their individual needs. As the number of partners, suppliers or products manufactured increases, these systems easily scale in response. And, with a single source of truth, pharma companies are able to ensure that each partner in the network is using the most up-to-date procedures.
2. Drive secure and efficient technology transfer
Technology transfer is a complex process, involving a large body of knowledge and multiple disciplines. Common challenges include missing critical content or providing methods that lack detail. These gaps can hinder a receiving manufacturing site from reproducing the results from the sending site.
With a consistent, controlled process, pharma companies can quickly locate and share all relevant information and be certain that their suppliers always have the most up-to-date content. Not only can suppliers access the right version, but the same document can also be securely shared across multiple suppliers — without them being aware of each other.
Looking ahead, pharma companies using cloud systems can transfer technology more collaboratively by allowing partners to provide feedback on procedures and protocols in real time. Questions from a new supplier or CMO can also be tracked to make sure they are addressed. Increasing collaboration and the addition of a feedback loop will help improve efficiency of all future technology transfers.
3. Systematize batch record review
Maintaining oversight of partner-generated information can be a challenge. Companies that modernize their batch and lot disposition process with partners remove the barriers to transparency for all parties.
For example, once a CMO uploads analytical test results or a completed batch record, the pharmaceutical company’s quality team can be automatically notified to review the data, and track questions and discrepancies. The CMO then addresses the issues, and if necessary, sends a revised version of the record for acceptance. This process adds control and reduces risk by seamlessly tracking and addressing all items that could escalate into product failures.
Using a modern system to track the data associated with the batch review process, companies can also measure the level of engagement for each CMO or supplier. For instance, the quality team can track the number of discrepancies for each partner, including response time, to help measure performance. Establishing similar operational and performance metrics across a range of key areas can help pharmaceutical companies better identify areas for improvement.
Get started by prioritizing suppliers
Adopting modern systems and smarter processes within a company’s four walls is just as critical as ensuring key suppliers and CMOs do the same. However, even with the benefits of improved collaboration, reduction of silos, automation of business processes, and improved time to market, some suppliers are hesitant to collaborate within their customers’ quality management systems — uncertain about how this could impact their day-to-day operations.
Here are two easy ways to encourage suppliers and partners to modernize:
Demonstrate value and ROI: Suppliers will recognize the advantages of collaborative, best-in-class cloud technology only when their customers invest the time to demonstrate the value, efficiency, and operational benefits. For example, joint workshops, user conferences, and product demonstrations will go a long way to supplier adoption while fostering more productive relationships.
Best-in-class technology enables companies to eliminate the manual steps commonly used today to track supplier quality, such as transcribing phone calls into the system of record, inputting incident reports, emailing back and forth with suppliers, scanning documents and more. One pharma company found that their supplier quality engineers were spending up to four hours per person each day on administrative tasks. With new technology, companies can dramatically improve efficiency and free staff to focus on higher value activities such as supplier risk management.
Modern systems also automate the ongoing process of managing supplier scorecards. For instance, alerts and workflows can be automatically triggered when any supplier nears an unacceptable quality threshold. Drug companies can set pre-determined limits across various quality factors, and if breached, the system demotes that supplier from “approved” to “conditionally approved,” and prevents any further purchases until resolved. If needed, it can auto-generate requests for “for-cause audits,” ensuring all high-risk suppliers are closely monitored. This also allows companies to be more proactive in averting a potential disaster. The same is true for suppliers that are equally as vested in avoiding the financial and brand damage stemming from a major quality event in one of their customers’ products.
Historically, drug companies have only been able to react after discovering that an approved supplier slipped from only one incident per quarter to 10 per quarter. By then, the damage has already been done and the costs are significantly higher than if the incident had been caught early. Modern solutions track suppliers in real time, empowering companies to head off a problem early and affording them more control for creative problem resolution. Suppliers, in turn, will likely reduce their quality infractions, and be rewarded by the drug company upgrading them to preferential status. Frequency and intensity of supplier audits can also decrease depending on consistent, positive audit results, scorecards, and risk ratings, translating into significant time and resource savings.
Simplify system onboarding: Naturally, some suppliers are reluctant to adopt a new system if it’s perceived as difficult to use. As consumers, we expect technology to be intuitive with clear, point-and-click navigation for rapid access to records, auto-populated fields to save time, and simple drop-down menus. The same is true in business, so it is important to select a system that is intuitive. According to Gilead’s partner survey, 100 percent of respondents said the solution (see sidebar on page 21) was easy to use, prompting two-times greater usage than expected.
Before implementation, pharma companies should help suppliers assess organizational readiness by identifying any requisite change management and evaluating the optimal training approach — both from a content development and delivery perspective — to ease the transition. By giving suppliers expert guidance before adopting a system, companies reduce any potential apprehension.
Post-implementation, pharma companies should provide system support in conjunction with their technology vendors. Experienced technology providers can provide additional virtual and/or in-person user training, educational materials, and troubleshooting and resolution to help onboard suppliers quickly and provide ongoing training sessions in regular intervals.
Modernize links in the supply chain
Modern technology is a key enabler to improving supplier quality management in the pharmaceutical industry. However, for drug companies to fully leverage the benefits of new technologies, it’s paramount to also include their suppliers and partners. Companies that demonstrate system benefits and incentivize suppliers by passing along benefits such as preferential status or less frequent audits (when possible) are likely to get the buy-in from key suppliers. Companies can also encourage system adoption by providing onboarding assistance and comprehensive, ongoing training.
The result is an increase in the quality of raw materials coming in from suppliers, which improves the quality of finished products going out to patients — the ultimate goal. Higher quality products reduce quality events while freeing resources to be redirected to other strategic initiatives like product innovation. This improves patient safety for existing products and also fuels accelerated development of more life-altering therapies for a win-win.
With 70 percent of manufacturing outsourced across more than 100 partners globally, Gilead Sciences required modern processes and technology across the organization, partners and supplier network to ensure effective quality management. Gilead selected a cloud-based quality management solution to harmonize business processes and collaborate more efficient across its globally dispersed teams.
Gilead worked with its technology partner to operationalize onboarding and ensure a smooth transition to the new solution. With all teams fully functioning, Gilead now has four-times more documents being shared in the system and significantly greater transparency across the entire enterprise. The company efficiently shares critical manufacturing content, data, procedures and other information with partners by connecting them to internal workflows in the system and, as a result, releases new products faster.