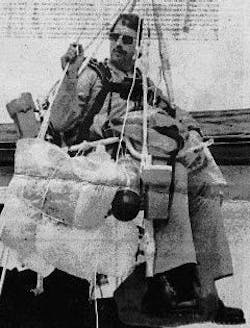
On July 2, 1982, Larry attached 42 helium-filled weather balloons to his Sears lawn chair, packed himself lunch and a beer, and took flight.
But “Lawn Chair Larry” apparently made some miscalculations when it came to scaling up the power of helium balloons and what it takes to initiate a smooth helium-fueled descent. His plan of leisurely drifting a few hundred feet above the Mojave Desert and then popping a few balloons with a pellet gun in order to land did not quite work out. Instead, Larry and his lawn chair ended up close to 16,000 feet in the sky, where he was spotted by airline pilots as he blew through controlled airspace, ultimately crashing down into (disabled) powerlines and landing himself in jail.
The pharma industry is no stranger to the complexities of scale-up. And as biologic drugs begin to dominate the market, manufacturers are finding themselves having to fundamentally reassess traditional supply chains, technologies, and operational approaches. This month’s cover story specifically addresses the unique challenges of scaling up production of gene therapies.
The promise of gene therapies is vast — they have the potential to cure previously incurable inherited diseases — from genetic disorders to cancer. Currently, U.S. approvals are limited, but the pipeline is brimming with gene therapy hopefuls and major players in pharma are investing heavily in the dream.
Determined to make their aspirations a reality, the gene therapy industry is hard at work figuring out how to make large-scale production run as smoothly and affordably as possible. What do those experienced in navigating gene therapy launches say is the key? Developing a comprehensive initial flight plan. Drug developers need to make decisions about how they want to scale up early in the process. This means seeking out the right technologies and finding (or building) the right facility.
This advice may seem difficult to act on when you consider how nascent therapeutic areas typically take off — a lot of hype followed by regulatory uncertainty, concerns over effectiveness and safety, and doubts about the ability to make products at a cost that’s profitable. But pharma has been here before (consider mAbs 20 years ago) and continues to make progress towards its end goal: a flexible platform for gene therapy manufacturing that companies can replicate.
The possibilities of gene therapies are sky high but success is predicated on a comprehensive scale-up and launch strategy — without that, your mission runs the risk of an unceremonious and costly crash landing.
About the Author
Karen P. Langhauser
Chief Content Director, Pharma Manufacturing
Karen currently serves as Pharma Manufacturing's chief content director.
Now having dedicated her entire career to b2b journalism, Karen got her start writing for Food Manufacturing magazine. She made the decision to trade food for drugs in 2013, when she joined Putman Media as the digital content manager for Pharma Manufacturing, later taking the helm on the brand in 2016.
As an award-winning journalist with 20+ years experience writing in the manufacturing space, Karen passionately believes that b2b content does not have to suck. As the content director, her ongoing mission has been to keep Pharma Manufacturing's editorial look, tone and content fresh and accessible.
Karen graduated with honors from Bucknell University, where she majored in English and played Division 1 softball for the Bison. Happily living in NJ's famed Asbury Park, Karen is a retired Garden State Rollergirl, known to the roller derby community as the 'Predator-in-Chief.'