Pharmaceutical manufacturing facilities rely on compressed air for many functions. Because of the risks associated with contaminated compressed air, manufacturers work to ensure the quality of their compressed air. Acceptable air quality levels can be determined using the International Organization for Standardization (ISO) 8573 standard for compressed air. ISO 8573 considers particles one of the major compressed air contaminants that can have an impact on end-products. Part 4 of the standard, which stipulates test methods for determining particle content, was recently revised in March of 2019. This revision included new requirements for particle sizing and counting instruments, clarification on the fundamental definition of particle size, expansion of what constitutes a particle, and changes to accepted testing methods.
This article focuses on the new ISO 8573 Section 4: Particle Content 2019 updates and particle analysis methods: light optical microscopy, scanning electron microscopy, and optical particle sizing and counting instruments.
ISO 8573-4: 2019 Particle Content Standard updates
Calibration requirements
In contrast to the previous edition, ISO 8573-4:2019 includes calibration requirements for optical particle sizing and counting instruments, commonly referred to as laser particle counting instruments (LPCs). LPCs must now be calibrated on at least an annual schedule and in accordance with ISO 21501-1 for optical aerosol spectrometers (OAS) or ISO 21501-4 for optical particle counters (OPC). These new requirements allow for more precise and accurate particle results from optical particle sizing and counting instruments.
Definition of a particle and particle size
ISO 8573-1 and ISO 14644 specifications have particle limits based on particle size ranges. Thus, the definition of particle size is paramount to consistently and accurately designating a particle purity class. The previous version of the standard only included information for sizing a particle based on its longest diameter, while the ISO 8573-4: 2019 revisions also include information for sizing particles based on a particle’s equivalent spherical diameter.
The equivalent spherical diameter is the equivalent diameter of a sphere that has the same properties as the particle being measured. LPCs size particles in this manner based on the scattered light properties of particles. These instruments are calibrated using spherical reference materials of a singular, known refractive index and size. These particle parameters may affect the scattered light properties of the particle and, therefore, the measured particle size by the LPC method. Therefore, it is important to consider these limitations of LPCs when selecting the appropriate particle content testing method. For some processes and/or applications, it may be imperative that particles are sized according to the longest diameter. Light optical or scanning electron microscopy would be suitable in this case.
Another major change from ISO 8573-4: 2001 to ISO 8573-4: 2019 is the expansion of what constitutes a particle. ISO 8573-4: 2001 had a limited scope in that it was only focused on determining solid particle content. This scope was expanded in ISO 8573-4: 2019 to also include liquid particles. This transition is in line with the standard pushing for LPCs as a main method option since these instruments do not differentiate liquid from solid particles. Distinguishing liquid from solid particles may be important in troubleshooting efforts as well as for facility risk assessments.
Method Updates
ISO 8573-4:2019 provides three analytical methods to determine particle content, including light optical microscopy, scanning electron microscopy, and optical particle sizing and counting instrumentation. The standard also presents guidelines on the range of particles able to be sized per method.
The ISO 8573-4: 2019 revisions narrow down particle testing methods to microscopy and LPCs, with added LPC calibration requirements aimed at yielding more accurate LPC particle results.
ISO 8573-4: 2019 particle analysis methods
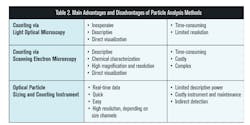
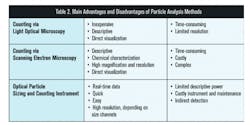
Each method described in ISO 8573-4:2019 has different advantages and disadvantages. Pharma manufacturers should consider which will most appropriately meet their needs in determining compliance to their desired specification.
Optical particle sizing and counting instruments (LPCs):
LPCs count pulses of scattered light from a single particle illuminated with a focused light beam. The magnitude of these pulses represents the sizes of particles. There are two types of LPCs discussed in the 2019 standard, optical aerosol spectrometers (OAS) and optical particle counters (OPC), as shown in Table 1.
OASs perform favorably over OPCs with regards to particle size resolution, with OASs typically having more size channels than OPCs. The resolution afforded to OASs may not be necessary for most applications. Both OPCs and OASs are suitable for determining ISO 8573 particle purity classes 1-5 for pharmaceutical manufacturers.
As stated previously, LPC instruments do not differentiate liquid from solid particle content and cannot determine these particle types separately. This can be a disadvantage when troubleshooting and diagnosing sources of contamination. However, liquid particles can be separately analyzed via microscopy using a sampling disc.
LPC analysis is advantageous over microscopy in its ability to provide real time particle results. Additionally, LPC analysis, unlike microscopy, does not require labor or time intensive procedures nor highly trained technicians. Thus, numerous samples can be taken in a short period of time over multiple sampling locations. LPC analysis can allow pharmaceutical manufacturers to troubleshoot and diagnose particle contamination quickly, potentially increasing the possibility of detecting and preventing contamination from reoccurring. Despite these advantages, LPCs are costly instruments, requiring frequent maintenance, and annual calibration. And, as discussed previously, calibration using spherical beads of a homogenous composition presents a limitation to particle sizing accuracy.
Along with their associated costs, LPCs can be sensitive and fragile instruments. Sampling dirty or moist air can damage and/or contaminate LPC instrument optics, resulting in costly repairs and lost testing-time. Microscopy may be the preferred method of analysis when determining particle content of contaminated air as sampling on a filter membrane greatly lowers the risk of damaging the testing equipment. When compared to the microscopy analysis methods, LPCs generally provide less information. LPCs display particle size data in terms of the counts per particle size range, dependent on the number of size channels, while microscopy can size particles individually. Furthermore, LPC analysis does not provide particle color, chemical composition, or shape, all of which are useful in determining the source(s) of contamination.
Microscopy:
ISO 8573-4: 2019 lists two microscopy-based methods, sampling via a filter membrane (or sampling disc) in conjunction with analysis via light optical microscopy or scanning electron microscopy. Microscopy, unlike LPCs, affords the opportunity to directly size, count, and visually describe particles. It should be noted that the microscopy-based methods are generally more time-consuming and can require highly skilled laboratory analysts. However, there are automated to semi-automated microscopy software programs on the market that can dramatically reduce the analysis time. These microscopy-based methods can be used to size particles according to the particle’s longest diameter.
Light optical microscopy is an inexpensive and simple method for counting and sizing particles. It is a suitable method for ISO 8573-1 particle purity classes 3-5. ISO purity classes 1-2 require quantitation of particles in the 0.1 µm to 0.5 µm size range, below the resolving power of light optical microscopy. Light optical microscopy is advantageous in that particles can be characterized according to color/translucence, shape, and topography. This information can assist in troubleshooting and combatting particle contamination by helping to identify the source of contamination. In many cases, light optical microscopy is an appropriate analysis method and represents an affordable option for pharmaceutical manufacturers to ensure the quality of their compressed air.
Scanning electron microscopy is a more advanced and complex method and generally more expensive than light optical microscopy. It can be used as the primary analysis method to size and count particles, in a similar manner to light optical microscopy, or can be used to further characterize particles. Scanning electron microscopy is a suitable method for ISO 8573-1 particle purity classes 1-5. This improvement in resolving power over light optical microscopy is due to the use of electrons, which have a wavelength of about five orders of magnitude smaller than the wavelength of light. Scanning electron microscopy also allows increased magnification, around 300 times that of light optical microscopy, which can allow for increased descriptive powers and identification of particles. One of the greatest advantages of scanning electron microscopy is the ability to characterize the chemical composition of particles through the use of an energy-dispersive (EDS) detector. Scanning electron microscopy’s ability to further characterize particles can provide key details for determining the source of particle contamination and subsequently reducing/eliminating that source.
In short, of the ISO 8573-4: 2019 listed methods, LPCs and scanning electron microscopy methods are applicable for ISO 8573 particle purity classes 1-5, while the light optical microscopy method is applicable for purity classes 3-5. Microscopy methods typically provide more detailed information relating to the particles analyzed, which can help resolve and prevent contamination issues.
In order to ensure the quality of the compressed air used in pharmaceutical manufacturing processes, many pharmaceutical companies test for particle content according to ISO 8573. The new ISO 8573-4: 2019 update adds stringent LPC calibration requirements, changes the definition of a particle to incorporate liquid particles, includes equivalent spherical diameter as a way to size particles, and narrows down analytical method options to microscopy and LPCs. It is important to understand the advantages and disadvantages of the three main particle analysis methods, light optical microscopy, scanning electron microscopy, and LPCs, in order to select the most appropriate method for the application in consideration (see Table 2).
Depending on a facility’s risk assessment and target specification/purity classes, light optical microscopy or LPCs may be the best option for primary particle analysis when considering cost and information gained. Subsequent particle characterization can be accomplished via scanning electron microscopy. Pharmaceutical manufacturers should choose an analysis method which can provide them with the information necessary to designate the particle purity class they require, per ISO 8573. It is often in the best interest of pharmaceutical manufacturers to test with a third-party, accredited laboratory. This ensures that analysts, testing equipment, methods, quality assurance, and testing data meet or exceed internationally accepted standards (e.g., ISO 17025 for testing laboratories).