Historically, pharmaceutical manufacturing has been associated with certain challenges. With traditional manufacturing, the pharma industry has been able to achieve a desired product quality but is associated with excessive costs, high waste, rejections and delays. Additionally, it lacks thorough understanding of the products and processes and proactive characterization of the failures. A thorough understanding of the drug product/processes will ensure robust and consistent quality. The U.S. Food and Drug Administration has given its guidance in 2004 to solve these issues under QbD.1 Implementing Quality by Design tools will help to alleviate the rejections, prevent the delays and reduce the associated costs.
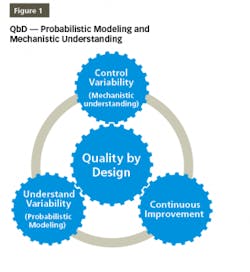
QbD is a disciplined and systematic approach for effectively developing and communicating product and process understanding. QbD provides the elements needed to generate data and extract information vital to building probabilistic and mechanistic models for product/process understanding and robustness, ensuring a high level of product quality and consistency, as shown in Figure 1. The probabilistic models should be supported by“The initial list of potential parameters can be quite extensive, but can be modified and prioritized by further studies (e.g., through a combination of design of experiments). Once the significant parameters are identified, they can be further studied (e.g., through a combination of design of experiments, mathematical models, or studies that lead to mechanistic understanding) to achieve a higher level of process understanding.”
This article discusses the role of mechanistic understanding complementing probabilistic modeling in QbD related to the pharmaceutical industry.
QbD AND MECHANISTIC UNDERSTANDING
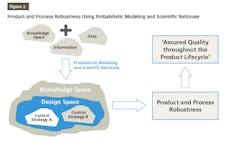
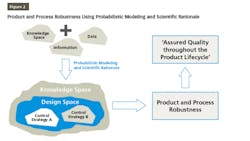
QbD is finding its place increasingly in pharma drug development and manufacturing. QbD is a comprehensive approach, and Design of Experiments (DOE) is one of the recommended tools used in the QbD probabilistic approach to build models for product and process understanding. These probabilistic models are built based on the data derived from observations and therefore, consists of analysis derived from the observed data. The probabilistic approach starts by defining independent variables. Based on well-characterized experimental studies in controlled conditions, an empirical equation is derived that is probabilistic and observation based. The model developed based on this empirical equation is reliable from the precision point of view but needs to be supported by scientific rationale for the sake of accuracy. Integrating probabilistic modeling and scientific rationale to build the design space and control strategy for a product will make it robust because it is supported by the scientific knowledge, characteristics of the product, and product processing mechanisms as well as the mechanisms by which different attributes and parameters affect the target product quality as shown in Figure 2. This results in ensuring that the quality of the products during their total life cycle is based on a scientifically sound understanding of the products as well as the processes.
Mechanistic understanding/scientific rationale provides a mechanism responsible for producing the patterns and/or processes that we see.2 It establishes the nature of the relationship, interactions and contributions of the critical attributes and parameters that give rise to the desired response factors. In other words, we can establish a cause and effect relationship that is not always feasible from a probabilistic model byIn a QbD approach, probabilistic modeling needs to be supported by mechanistic understanding. In this approach, the raw material characteristics and process parameters are related to critical quality attributes (CQAs) by assessing their criticality.5 Mechanistic understanding facilitates an approach to understand the risk perspective for quality assurance of the final drug product.6 According to the meeting report of the second FDA/PQRI conference, some case studies in the pharmaceutical industry identified the critical attributes and parameters by focusing on the mechanism based product and process understanding. These studies showed that when developing the control strategy, deeper mechanistic understanding of the product and process was associated with better results.9 Integrating mechanistic understanding with elements of probabilistic models ensures that the relationships are statistically significant and scientifically relevant as well.
CONCLUSIONS
Integrating mechanistic understanding in different areas of drug development and manufacturing will result in a thorough understanding of product and processes, and increased ability to consistently make a quality product. Probabilistic models contribute significantly to product and process understanding and are the cornerstone of quality products. However, at times, probabilistic models without scientific rationale can be incomplete. Probabilistic models and mechanistic models, in general, complement each other. But, in cases where mechanistic understanding doesn’t complement mechanistic modeling, credence should be given to mechanistic understanding because mechanistic understanding is based on scientific rationale.
Incorporating mechanistic understanding to support the probabilistic modeling approach is required for effective implementation of QbD. Probabilistic modeling supported by mechanistic understanding will lead to a reproducible, robust and accurate approach providing complete assurance of the desired quality.
*Author titles: Rupninder Sandhu, Senior Scientist, Global Quality Analytics; Barry Gujral, Director, Global Quality Analytics; Andrew McNicoll, VP, Global Quality Systems and Compliance; Pharma Services, Patheon, part of Thermo Fisher Scientific
Additional contributing authors include: Karlheinz Giselbrecht, Group leader, R&D, Linz Site; Martina C. Kotthaus, Head of R&D, R&D, Linz Site; Pharma Services, Patheon Austria GmbH & CoKG, part of Thermo Fisher Scientific.
Industry correspondence for this article provided by: Ajaz S. Hussain, Insight, Advice and Solution; Former Director, FDA
For additional information contact Barry Gujral: [email protected]
References
1. Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; September 2004.
2. Gujral B, Amanatides P; Pharma QbD 2012; Transdermal Drug Development using Quality by Design.
3. Yu LX, Baker J, Berlam SC, Boam A, Brandreth EJ, Buhse L...Vaithiyalingam S. AAPS J. 2015 Jul; 17(4):1011-8. doi: 10.1208/s12248-015-9754-4. Advancing Product Quality: a Summary of the Inaugural FDA/PQRI Conference. Epub 2015 Apr 4.
4. FDA Pharmaceutical Quality Oversight; accessed October 17, 2017
5. Yu LX, Akseli I, Allen B, Amidon G, Bizjak TG, Boam A...Zezza D.; Advancing Product Quality: A Summary of the Second FDA/PQRI Conference; AAPS J. 2016 Mar;18(2):528-43. doi: 10.1208/s12248-016-9874-5. Epub 2016 Feb 9.
6. QbD: The Role of Mechanistic Modelling; Sept. 2008.