Granulation processes are used routinely across a number of industries to transform the properties of powder blends, often with the aim of producing an optimized feed for subsequent processing. High shear wet granulation (HSWG) is a flexible, efficient and reproducible technology and the preferred choice for many pharmaceutical applications. Combining short processing times with the capacity to deliver dense, uniform granules, HSWG is particularly suitable for producing an optimized feed for tableting and is an integral step in many oral solid dosage manufacturing processes.
An important aspect of current efforts to transform the efficiency of pharmaceutical manufacturing is the identification of Process Analytical Technology (PAT) that supports the better understanding, monitoring and control of key steps such as HSWG. This is especially true as the industry embraces continuous manufacturing, which tends to be associated with higher levels of process control than batch. HSWG monitoring and control is complicated by the fact that granules are typically an intermediate within a process, rather than the end product. Learning how to control the critical process parameters (CPPs) of a HSWG process so as to produce a tablet with defined critical quality attributes (CQAs), after several subsequent stages of processing, is a significant challenge.
In this article, we consider the process of high shear wet granulation, the benefits associated with it - particularly within the context of tablet manufacture - and strategies for characterizing the resulting granules. A particular focus is the use of analytical techniques, including new technology for continuous, in-line measurement, that characterize bulk properties of the powder rather than properties of the constituent particles. Such technologies have been shown to measure properties that correlate directly with finished tablet quality highlighting their value for HSWG monitoring and control.
BENEFITS OF GRANULATION
One of the primary reasons to granulate a fine powder blend is to improve flowability. Fine powders are usually relatively cohesive, exhibiting poor flow properties that can compromise performance in downstream processing steps or during product use. Larger granules, in contrast, tend to flow more freely delivering greater manufacturing efficiency. Beyond this, granulation delivers a range of other valuable benefits that include:
- Enhanced homogeneity and a reduced risk of component segregation. This can be particularly helpful in ensuring the content uniformity of a finished product, for example, in tableting blends containing low levels of an active pharmaceutical ingredient and/or very fine APIs dispersed in much coarser excipients.
- Denser particles with a lower packed volume. Larger, denser particles can pack efficiently relative to finer, more cohesive ones which trap air. Granulation can therefore reduce storage volume needed for an equivalent mass of product.
- A reduced dust hazard. Reducing dusting can be an important health and safety gain, particularly when dealing with high potency APIs.
- Improved compressibility. Engineering an optimal level of compressibility into a granular feed can directly enhance its performance in subsequent processing steps.
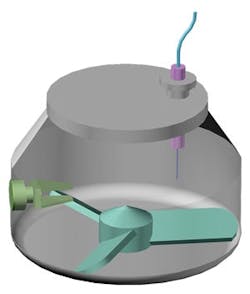
A schematic of a high shear wet granulator showing impeller, chopper and inline sensor for process monitoring.
Beyond these generic benefits, HSWG offers further advantages for a number of pharma applications. Like other wet granulation technologies, it is suitable for a wide range of materials and for almost any drug dosage. It offers short processing times, minimal binder use, and enables the granulation of certain types of highly cohesive material that cannot be successfully processed with low shear techniques. Relative to low shear processes, HSWG also produces denser, less friable granules. These advantages help explain the popularity of HSWG within the industry, however, its practical implementation can be challenging.Though HSWG is an inherently simple process, it presents two major challenges that impact practical implementation, the first of which is the difficulty of scale-up. Differences in equipment geometry and/or process dynamics from scale-to-scale complicate the transfer of optimized processing conditions. For example, a large scale unit may need a water addition of 22 percent to reach the same endpoint that a smaller scale unit reaches with a water addition of 26 percent. This is a big issue in the development of batch processes, which may go through several scales of operation prior to commercialization, and could complicate the early definition of design space.
The other challenge is accurate endpoint detection, identification of the point at which the granules have reached a state that is optimally compatible with their intended application. Here problems arise because granules are an intermediate, rather than the product of interest, in the majority of applications. In tableting, for example, developing correlations between CPPs for the HSWG process and tablet quality relies on the implementation of a statistical design of experiment (DoE) study that involves processing granules through the tablet press and assessment of the CQAs of the finished product (e.g. assay, weight, hardness, dissolution and disintegration). This lengthy, iterative approach is sub-optimal from the perspective of efficient process development and scale-up, and ultimately from the perspective of ongoing optimization and/or control of the HSWG.
The identification of PAT that is able to measure a parameter, during granulation, which securely and relevantly quantifies the quality of the exiting granules offers the opportunity to streamline the development process. To support the use of HSWG as a precursor to tableting, this requires PAT that is able to measure variables that correlate directly with the CQAs of the finished tablet, thereby eliminating the need for full product work up to assess granule quality.
SELECTING PAT FOR HSWG
When considering how best to characterize the product from an HSWG process, one approach is clearly to measure discrete properties of the granules themselves such as a particle size. Typical aims of the HSWG step within a tableting process are to improve blend flowability, reduce/eliminate segregation, and enhance compressibility so as to ensure high throughput in the press and target product quality. Particle size may impact all of these behaviors, but so do many other parameters. The flowability of powders, for instance, is a function of many different properties of the constituent particles, including shape, density, surface texture, porosity and hardness/friability, all of which may be impacted by granulation conditions. Looking at just one influential variable may therefore be less than optimal when it comes to establishing robust correlations with performance in the press and with tablet quality.
An alternative strategy is to directly measure bulk powder properties such as flowability of the granulating mass or the exiting granules. Dynamic powder testing is an at-line technique that quantifies powder flowability by measuring the axial and rotational forces acting on a blade as it is rotated through a powder sample. With dynamic testing, dry or moist powders can be measured in a consolidated, moderate stress, aerated or even fluidized state to simulate the process environment and generate highly relevant data. Furthermore, in addition to flowability, dynamic powder testers are able to measure other bulk powder properties that are directly relevant in quantifying granule quality. These include compressibility and permeability.
Experimental studies using HSWG to prepare optimized blends for tableting have shown that the properties of finished tablets can be predicted from dynamic measurements of the wet granules. A direct relationship was observed between the basic flowability energy (a measure of confined flow properties in a low stress state) of the wet mass and of the dried granules, and tablet hardness. This work clearly highlights the potential for bulk powder measurements to fulfill the defining requirement for PAT for HSWG processes. The introduction of new, equally successful techniques for in-line, real-time measurement that operate on closely similar principles is therefore an exciting development.
A New PAT for HSWG
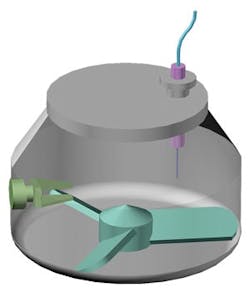
Schematic of a drag force flow sensor for the real-time measurement of flow forces within in-process material.
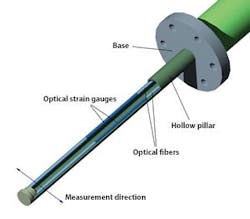
The key features of a drag force flow sensor for in-line granulation monitoring are shown in Figure 2. An optical sensor interrogator processes and analyzes the signal from the sensor, and complementary temperature measurements are made to enable the automatic correction of any temperature-related drift in the measurement baseline. The sensor typically has a diameter of just 1-4 mm and can be mounted directly inside a granulator.Material flowing past the sensor causes a deflection, the magnitude of which is precisely measured using the sensitive fiber-optic strain gauges on its inner surface to generate Force Pulse Magnitude (FPM) data. In a granulator, FPM peaks with each passing of the impeller blade giving a sinusoidal signal. Filtering and averaging of this signal yields a smoothed FPM data stream that provides robust and highly sensitive measurement of the flow forces within the in-process material. These dynamic real-time measurements of the granulating mass correlate directly with properties such as granule size and density, which change during the course of a HSWG.
In principle, this approach is akin to using the power drawn by the impeller motor for granulation monitoring - a traditional practice at the commercial scale - but it is orders of magnitude more sensitive. The practical benefits of the technology include:
- Minimal intrusion into and disruption of process flow
- Differential measurement that is not subject to baseline drift
- Relative insensitivity to the adherence of process material on the sensor surface
- High reliability/minimal maintenance (no moving parts)
- High frequency measurement rates (up to 500 samples per second) for real-time monitoring
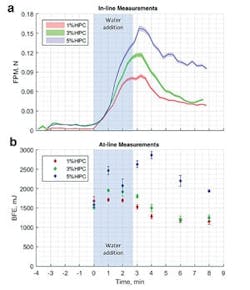
An in-line drag force flow sensor (a) tracks the progression of HSWG processes in an analogous way to at-line dynamic powder flow measurements (b).
Figure 3 shows data measured using an in-line drag force flow sensor (Lenterra Flow System, Lenterra Inc.) to monitor the progression of HSWG trials carried out to produce three batches of a placebo pharmaceutical formulation containing different levels of hydroxypropyl cellulose (HPC). The addition of water to the dry blend is associated with a pronounced increase in FPM, which peaks shortly after the end of the water addition period highlighting the endpoint of the granulation which is conventionally recognized as occurring shortly after water addition is complete. The data clearly differentiates the granules produced at 1, 3 and 5 percent HPC and shows that higher binder contents lead to the formation of stronger granules.For comparative purposes, BFE data measured during the same trial using a dynamic powder flow tester (FT4 Powder Rheometer, Freeman Technology) are included (see Figure 3). These results show similar trends to those observed in the FPM data indicating that, like dynamic powder testing, the in-line drag force flow sensor offers the ability to measure the granulating mass in a way that directly correlates with the quality of tablets produced from them. This highlights the technology’s significant potential as a real-time, in-line PAT that can be used in an analogous and complementary way to at-line dynamic powder testing to optimize HSWG processes.
PROMISE OF NEW TECHNOLOGIES
A primary aim of the PAT initiative was to encourage the pharma industry to embrace innovative technology in order to reach new levels of manufacturing efficiency. Identifying the most effective analytical techniques for the monitoring and control of critical processes such as HSWG is vital for efficient process development, scale-up, monitoring and control, and remains an ongoing task. Though widely used within the industry, HSWG processes do not scale-up easily and can be difficult to control effectively.
New in-line technology that enables the real-time measurement of flow forces within a granulating mass can be highly effective for monitoring granulation and shows significant potential for sensitive, accurate, real-time endpoint detection. Rather than relying on a classical approach of measuring a single granule property, this technology quantifies the flow behavior of the powder bulk as a function of process variables such as water content, formulation or granulation conditions. In this respect it is highly complementary to at-line dynamic powder testing, which has already been shown to have significant value for the optimization of HSWG processes, particularly within the context of tablet manufacture. By delivering data that can be directly correlated with the CQAs of finished tablets, these technologies offer opportunities to streamline and accelerate the development of both batch and continuous tablet manufacturing and, going forward, to directly support their advanced control.
REFERENCES
- Handbook of Pharmaceutical Granulation Technology, 3rd Edition, ed D. M. Parikh, Informa Healthcare, 2010
- Freeman, T. (2014) Choosing a Powder Tester. Freeman Technology e-book.
- Freeman, T. (2014) In Pursuit of Wet Granulation Optimization. Pharmaceutical Manufacturing.
- Narang, AS. (2016) PAT for High Shear Wet Granulation: Wet Mass Consistency Reported by In-Line Drag Flow Force Sensor Is Consistent With Powder Rheology Measured by At-Line FT4 Powder Rheometer. Journal of Pharmaceutical Sciences. 105:185-187.