In the multidisciplinary pharmaceutical industry, photonics plays an increasingly crucial role in the manufacturing process. From quality assurance to marking, the scope of photonics is wide-reaching. Specifically, the use of lasers and machine vision in oral solid dose (OSD) manufacturing is revolutionizing functionality, security and speed while reducing costs and boosting efficiency.
In certain regions of the world, a growing problem concerns medication quality, stemming from poor manufacturing processes. For example, drugs manufactured with a lower dosage of active ingredients than approved by regulators is a dangerous situation that can lead to patients not receiving a medication's full benefit. Worst-case black market scenarios[1] involve nonpharmaceutical-grade or harmful ingredients resembling medication being used as substitutes, such as chalk and talcum powder.
The gray market,[1] on the other hand, manifests itself in several ways, but with reselling, importing and exporting that ultimately can have high costs both for the end consumer and the manufacturer. ("Gray market" goods are also termed parallel imports. This is when someone other than the designated, exclusive importer buys genuine trademarked goods outside the U.S., for example, and imports them for sale in the U.S., in direct competition with exclusive U.S. importers.[1])
Gray market companies acting as secondary wholesalers are a growing problem within the industry, as well. When a pharmaceutical wholesaler sells to a secondary wholesaler, limited-availability prescription drug pricing can become inflated, with pills passing through the hands of numerous wholesalers.[2] In anticipation of a shortage, these gray market companies incite panic and are able to resell drugs to consumers at higher prices.[3,4]
Pharmaceutical manufacturers should express concern over the gray market, particularly since it most commonly affects their bottom-line profit. When medicine is inexpensive in less-developed countries but moves through the gray market to be resold in countries without drug pricing regulation, such as the U.S., the availability of cheaper drugs in developed markets can take away from a manufacturer's margins and profit.[5] Laser marking of unique product identification, both overt and covert, helps control and promote an orderly supply chain.
LASERS FOR PHARMA
Lasers now are used in several ways for pharmaceuticals, particularly where traceability and security are important considerations. Packaging is being laser-marked with a unique identification number, allowing for manageable tracking through the supply chain. A given product can be traced to a specific manufacturing facility, batch number or manufacturing date, providing transparency and accountability and ultimately boosting consumer confidence. With OSD tablet marking, high-speed lasers have the capability to mark on the fly with a unique identification number, if desired – even down to individual tablet level – without slowing down process speed.
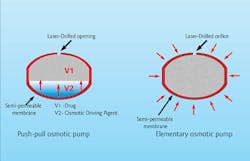
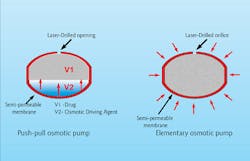
An osmotic pump drug delivery system.
For many years, lasers have been used for tablet drilling, with one of the most important applications of this technology being the osmotic controlled-release oral delivery system. This technology uses osmosis pressure as the driving force to dispense medicine in a controlled time-release manner.[6] This drug delivery system, in the form of a tablet, usually consists of a semi-permeable outer membrane, the medicine and an osmotic core as the driving force that delivers medicine through the laser-drilled hole.Several other methods for time-release drug delivery exist, with the commonality being a semi- or insoluble outer layer and either single or multiple laser-drilled holes. Recent years have seen a significant increase in demand for improved manufacturing methods for time-controlled drug delivery systems. With the expiration of several primary patents for osmotic pump delivery, the adaptation of this technology has grown. Laser drilling also is used in fast medicine release, not only in slow time-release. In this method, a tablet's protective outer layer is laser-drilled with a multi-hole pattern, causing the outer shell to collapse once the pill comes into contact with water in the digestive system.
Data-matrix code, laser-inscribed on gelcaps with encrypted manufacturing information. Images courtesy of PhotoScribe.
As with tablet drilling, direct laser marking has become an important application for lasers in the pharmaceutical industry. Before lasers, OSD forms were traditionally imprinted using one of two methods.[7] In the first method, a mark is molded into a tablet during the compression of the powder in the manufacturing process. This method is limited, however, as it allows only for marks that are simple and large in composition, usually containing two to five characters or an elementary symbol. The second traditional method is offset rotogravure printing, which has significant drawbacks including cleanliness of operation, the use of solvents and extra downtime to clean and maintain equipment. Overall print quality can also be limited.Laser marking was introduced to combat these problems. To accommodate the large volume usually required by pharmaceutical manufacturers, the mark-on-the-fly method is well-suited, during which the laser follows the product on the production line and marks with precise placement, allowing for continuous marking at extremely fast speeds with rates of up to hundreds of thousands of tablets per hour. The most common setup for such an operation includes a quadrature encoder, which gives the controller data on the conveyor speed and direction. In addition, a sensor mounted on the conveyor gives the controller a trigger signal that tells the laser when to fire.
Several types of sensors are in commercial use today, including inductive, photoelectric, fiber optic, ultrasonic, magnetic and camera-based vision systems. Some sensors can identify not only the location of the conveyor but also the presence of the OSD form, directing the laser to fire when the tablet is properly placed. The combination of both devices allows for real-time feedback, ensuring accurate mark placement on the pill. In high-speed laser marking, delays of only a few microseconds can make the difference between a successful mark and a failed one. However, with correct and careful selection of system components, these systems have been shown to be extremely robust and highly reliable.
When considering color in OSD marking, laser wavelength and optical beam delivery are carefully selected based on the type of tablet being marked. In most cases, OSD's interact with a laser producing sufficient contrast. However on other dose forms like transparent gelcaps, laser markings appear to be a white, frosted color. Several methods can be employed to enhance this contrast or even to introduce contrasting color, if desired.
In one method, FDA-approved laser-sensitive additives are combined during the manufacturing process, allowing production marks based not on the ablation of the dose form, but rather on a thermal chemical reaction with laser energy. This method allows the usage of lower energy density, sufficient for the production of high-contrast, high-resolution markings without material ablation.
In another method, the tablet's protective outer layer comprises multiple layers of different colors, and the laser selectively ablates the outer layer, revealing the contrasting color below. This method allows the mark to appear in color, rather than being limited to the black or white usually associated with laser marking.
Laser in place to inspect tablets as they are conveyed to their next processing step.
Lasers have the significant advantage of being a non-contact marking technology, reducing contamination, breakage and rejection rates. In addition, no tools or dies need to be changed, as the indicia are computer- generated and can be modified easily. Because of laser marking's advantages as a non-contact printing method, older equipment in the industry could, in many cases, be retrofitted with lasers, adding capabilities to machines currently in use.Pharmaceutical companies are taking advantage of the technology also to imprint branding on a pill. The logo or alphanumeric or another identifying mark adds functionality beyond the rudimentary FDA requirements.
Advances in laser technology and beam-delivery systems make it possible to mark on the fly with serial numbers, batch numbers or logos down to a single-unit basis. FDA regulations do not allow drug products in solid oral dosage form to be introduced (or delivered for introduction) into interstate commerce unless they are clearly marked or imprinted with a unique code. In conjunction with the product's size, shape and color, this code permits the unique identification of the drug product and its manufacturer or distributor. Product identification requires that active ingredients and dosage strength be documented.
Machine-readable code, such as data matrices, can also be marked onto dose forms and used for automated identification. Codes on pharmaceutical packaging identify the manufacturer, date of production, expiration date, and serial and batch number, allowing for full transparency in production. Encrypted information could be connected to a database that would follow a pill along its supply chain travels. This tracking system could help battle the huge global problem of counterfeit medicine, which has resulted in fatalities.
MACHINE VISION FOR PHARMA
Laser-based machine vision technology truly automates pharmaceutical manufacturing, making the process systematic, highly efficient and cost-effective. Lasers significantly increase production uptime, streamline the process and reduce costs, all while delivering new and improved capabilities ranging from product identification to functionality.
Adding identifying marks and barcodes to tablets, other dose forms and packaging is important, but without automated inspection and quality assurance, this process would be inefficient. Across companies, machine vision systems are being added to printing devices, ensuring that dose forms are complete and unbroken, while also verifying print quality.
Pharmaceutical-adapted machine vision cameras are mounted on printing devices to inspect pills in real time. Cameras are mounted on production lines for several purposes. First and foremost, the camera inspects the pill's integrity. If the pill is incomplete or broken, machine vision will send a signal to initiate a burst of air that can push any single pill toward the rejection bin (several rejection mechanisms exist, but the burst of air is the most popular).
A camera also is used to verify that tablet marks are correct and of good quality, again sending a command to reject any single unit that is not up to standard. For tablets marked with a unique identification, this scan is also the first in the product's supply chain tracing. Information about the tablet, such as its manufacturing date, batch number and factory location, is saved to a database.
Machine vision also can analyze shape and color to identify proper placement. For example, on a bicolor tablet where only one of its colors should be drilled, machine vision identifies the color and sends a command for the laser to mark the correct side. From capturing an image on camera, to analyzing product features, to making decisions based on predefined criteria and information sent to multiple devices (such as rejection/ejection and laser marking), complex interactions such as these are done in real time. In a cycle as short as microseconds to a few milliseconds, a pill is instantaneously and seamlessly processed.
REFERENCES
1. Fish and Richardson. Counterfeiting and the grey market. http://www.fr.com/files/uploads/FINALCounterfeitingtheGreyMarket1.pdf.
2. M. Tomsic (March 2013). Gray market companies take advantage of drug shortages. WFAE 90.7. http://wfae.org/post/gray-market-companies-take-advantage-drug-shortages.
3. M. Cohen (August 2011). Pharmaceutical gray market thrives along with national drug shortage. Philly.com.
http://www.philly.com/philly/blogs/healthcare/Pharmaceutical-gray-market-thrives-along-with-national-drug-shortage.html.
4. J. Lubell (August 2014). Lawmakers hear evidence of gray market in shortage drugs. Amednews.com.
http://www.amednews.com/article/20120820/government/308209957/4/.
5. Quantiz. Regional pricing and the grey markets.
http://www.quantiz.com.br/artigos/regional_pricing_and_the_grey_markets.pdf.
6. Process for forming outlet passageways in pills using a laser. U.S. assignee, Patent 4088864 A. N.d. Print.
7. U.S. Food and Drug Administration CFR - Code of Federal Regulations Title 21. See Sec 2016.10, Code
Imprint Required.
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=206&showFR=1.