Pharma is known for its late adoption of new technologies. The industry thoroughly studies risks, pros and cons, etc., before adopting any new technology. But now as companies are being compelled to streamline their process and reduce costs, they are finding new ways to optimize complex processes by adopting cloud computing. The benefits of the cloud “pay as you go” model, low capital investment and low run costs will drive innovation in products and bundled services quick to the market[1].
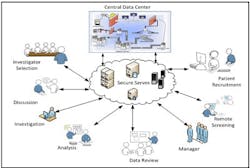
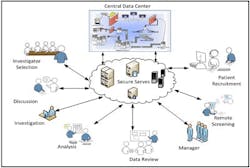
SaaS solution for Clinical Trial.
Over the last 10 years the technology industry has changed dramatically; there is advancement in technology, but the manufacturing industry is facing business challenges centered on improving performance of applications and infrastructure while controlling the cost of doing business. One ray of hope for controlling industry’s mounting challenges is Cloud computing. Pharma enterprises are trying cloud analytics, automation and marketing, and exploring the benefits of emerging technologies [2].Cloud software solutions are taking on several different tasks in the pharmaceutical industry. Its implementation can mean improving the quality of data to support sales, or providing practical ways for clinical trial site managers to communicate across wide geographic divides. It’s also making it easier for companies to merge without the problems that invariably arise when data between disparate computer systems is aggregated [3]. But looking ahead, there are also some intriguing applications that involve combining the cloud with big data from public and private sources and applying analytics.
To make the best use of the existing systems, sales force need most up to date and accurate information about stocks of the product, physicians contact details, social media networks they favor and whether their practices are accepted and very importantly help in avoid duplications.
In addition sales force, shares databases that are helping drug developers and manufacturing identify principal investigators. Principal investigator contact details used to be considered proprietary information. Regardless, there are a number of risks and challenges Pharma faces when it comes to adopting cloud-based computing solutions:
- Data/Information Security (VPN and Encryption) because the industry is highly regulated
- The other risk need to be considered in service provider as they don’t understand Pharma security[4] and regulatory requirements
- Data migration problems when changing the cloud provider (security validation, etc.), since the validation of the system involves rigorous process
- Surveillance of the internet, due to spying on people private data, the regulatory agency will come under pressure to do more to protect' private data. The agency will tighten data protection laws. So they will come out with strong solution information technology industry can offer alternatives ideas to be explored, like secure cloud computing and better links between technologies.
- Requirements on GxP -The service provider provides capabilities to facilitate the validation of Pharma specific solution using the cloud service. The validation remains accountability of Pharma and needs to be defined in a validation plan[5].
- Risk Management - Vendor transparence and audits are “must.” Legal, compliance, and reputation risks. Cloud vendors leaking, breaching, losing, damaging, or impeding access to various types of sensitive or valuable information.
- Operational risks - IT security, performance, or availability with strong encryption, access control, monitoring, and physical separation of resources.
- Security incidents - Most logs and documentation is in the control of the cloud provider. The cloud user will rarely have access to this data to determine security incidents.
- In shared environments the irreversible erasure or anonymization of data is difficult and since the cloud user has not access to the computing environment, it cannot be verified.
MITIGATING THE RISK AND ADOPTING THE CLOUD
Those in Pharma looking to adapt cloud technologies can avoid risks by setting clear Qualification and Validation goals can to help manage these risks and provide auditable evidence of how this has been done.
Best Practice for risk mitigation include:
- Data protection and encryption
- Segregate operations with respect to GxP, SoX, business confidential data
- Regulatory compliance
- Virtual Machine level security
- The GAMP (Good Automated Manufacturing Practice) forum could provide guidance on Infrastructure Qualification, as well as validation of applications risks around security need to be identified, managed and documented
- Differentiate the regulatory and security requirements to manage financial, legal and IP data from what the regulators require of GxP
- To maximise effectiveness and minimise risk (and ultimately cost), security and privacy must be considered from the outset of any cloud implementation not after implementation and deployment
- There should be careful evaluation of sensitivity of data being managed and its security (Like clinical trial data) with due consideration
- The Service provider needs to be frequently audited, like any other contractor, to have quality of service & well maintained documentation with respect to GxP requirements.
CLINICAL TRIAL SUCCESS
Clinical trials are an organized set of tests in medical research and drug development conducted on human beings to provide adequate information on its intended use as a therapeutic agent including its safety, efficacy data, and adverse effects on human-beings[6]. Clinical trials are conducted only after satisfactory data has been gathered on the quality of the nonclinical safety, and health authority/ethics committee approval is granted in the country where approval of the drug or device is sought. A trial must be conducted according to a protocol that describes the objective, design, methodology, statistical considerations, and organization of the trial. An electronic document designed to record all of the protocol-required information to be reported to the sponsor on each trial subject. During clinical trial data privacy of the patients to be maintained as per Good Clinical trial Practice
ELECTRONIC DATA CAPTURING SYSTEMS
Case report forms are manually filled at site and mailed to the company for which trial is being performed. The data on forms is transferred to the Clinical Data Management System (CDMS) tool. The most popular method being double data entry where two different data entry operators enter the data in the system independently and both the entries are compared by the system. In the case where the entry of a value conflicts, the system alerts and a verification can be accomplished manually. The data in CDMS are then transferred for the data validation.
Many drug manufacturers are using Web-based systems for capturing, managing and reporting clinical data. This not only helps them in faster and more efficient data capture, it speeds up the process of drug development.
SCALABLE, COST-EFFECTIVE CLINICAL RESEARCH SERVICES
Previously, enterprises had to buy or build and maintain sophisticated IT infrastructures, incurring great costs in the process and required to support all kinds of application from web to legacy systems. Software-as-a service (SaaS) computing also can provide an effective alternative, and enterprises can now plug-in and utilize software services whenever they need them, utilize them for as long as they need them[7].
A system which can deliver clinical solution as a SaaS with an on demand approach is best suited for the pharma needs. This system which can rapidly and efficiently deliver the core value of the clinical research application with the ability to capture, manage, share efficiently and effectively across thousands of investigator and among all stakeholders will help reduce the time for the whole clinical research cycle.
Employing the SaaS model can help reduce necessary investments, dedicated IT resources and other expensive IT infrastructure. With the inherent features of software solutions distributed in the cloud, one can expect the following advantages:
- On Demand Service and across geographic areas
- Dynamic computing resources
- Rapid Scalability
- Lowered operational costs
- Increased security such as PCI DSS, HIPAA, SOC2
- Measured Services
- Fault tolerance
- Disaster recovery & Business Continuity
- Uptime
There are various advantages for the pharma and biotech industry in utilize Cloud computing, it is about choosing the right solution which can offer them the balance of data protection, structuring and maintained of data.
KEY BENEFITS
By providing seamless and transparent integration with database, middleware, web frameworks, security and virtualization services. PaaS significantly improves developer productivity by removing integration challenges inherent in traditional software development model. Additionally, shorter software development lifecycles or faster delivery times are possible with PaaS since software development and testing can be both performed on a single PaaS platform as opposed to maintaining separate development and test environments for in-house software development
Dynamically scale up or scale down computing resources with changing demand levels. This ability to rapidly configure and scale computing, storage and other infrastructure resources on-demand, as opposed to buying more infrastructure capacity than needed upfront in anticipation of future growth, is what makes this delivery model so compelling in nature.
SaaS allows companies to do what they are good at, i.e., research, develop, and manufacture medicines to manage applications and data. But SaaS can’t evaluate, manage suppliers and hold them accountable for fixing problems. As with most all regulated computing applications, i.e., 21 CFR Part 11, electronic records are to be as trustworthy, reliable and generally equivalent to paper records. Classifying the data into Infrastructure-as-a-Service (IaaS), Platform-as-a-Service (PaaS) and Software-as-a-Service (SaaS) will benefit the pharma industry at large.
REFERENCES
[1] Infosys Point of view on Pharam& Life Science: http://www.infosys.com/industries/life-sciences/white-papers/
[2]Pharma and Life Science Trends: http://www.infosysblogs.com/lifesciences/
[3]Cloud computing drives faster innovation: Matt Wood :http://clouduser.de/en/gastbeitraege/cloud-computing-drives-faster-innovation-in-life-sciences-6777
[4]Security in cloud what it means for Pharam: http://www.informationweek.com/qanda-eli-lilly-on-cloud-computing-reality/d/d-id/1094101
[5]Barriers to Cloud Adoption and Overcoming the Challenges :http://www.samedanltd.com/magazine/11/issue/164/article/3109
[6]Detailed overview of Clinical Trial: http://en.wikipedia.org/wiki/Clinical_trial
[7] Hype Cycle for Life science 2013 : Gartner report : https://www.gartner.com/doc/2570915/hype-cycle-life-sciences-
ABOUT THE AUTHORS
Raju Singitis is a senior consultant with Infosys. He works as part of Pharma Services unit within Infosys. He has around 22 years of Software industry experience and involved in consulting pharma solutions for various customers with varying scale and size. He can be contacted at [email protected]
Sridhar Murthy is a senior architect with Infosys. He works as part of Engineering Services unit within Infosys. He has around 16 years of Software industry experience and involved in Enterprise architecting for various customers in Cloud computing and virtualization. He can be contacted at [email protected]