Groundbreakers: Tomorrow's Drug Manufacturing Facilities
2012 marks what some analysts are already expecting to be the most challenging year in the pharmaceutical industry’s history. As patent expirations and global competition step up, and the mantra changes from blockbuster to nichebuster, manufacturers are chipping away, wherever they can, at the years and millions of dollars required to launch each new product. If they must fail, more pharmaceutical manufacturers acknowledge, they’ll need to fail fast and move on quickly.
| Groundbreakers: Related Articles |
Pharma’s older infrastructure has not always allowed this to happen. Over the past few years, the pace of pharma plant consolidations and closures has increased. In the U.S. alone, 38 drug manufacturing facilities were shut down last year, and 65 the year before, according to Pharmalot’s Ed Silverman, who analyzed data from the Sugarland, Texas-based research firm, Industrial Information Resources, in December. However, he notes that 106 new plants and laboratories, worth an estimated $4.3 billion in contracts, are now planned or under construction.
Companies in pharma’s newest sector, biopharmaceutical manufacturing, are leading the way in harnessing technologies and methods to reduce the timeline and risk of building facilities to make new products. This will be important, says BioPlan Associates principal Eric Langer, since biopharm companies will be increasing production capacity by 25%, globally, over the next few years. Biosimilars promise to intensify the competition, and companies are gearing up for production in flexible facilities throughout the world. Enabling pharma facilities’ transformation are modular construction, which has been around for decades but is now possible in shipping-container-sized units, as well as disposable bioprocessing equipment, standardized solutions allowing for continuous operation and quick validation.
At the same time, modeling and simulation tools are becoming more important, particularly for training and startup, as are standard templates for IT and process control. For MedImmune’s new biopharmaceutical manufacturing facility in Frederick, Maryland, which won ISPE’s Facility of the Year competition last year, best IT and automation practices not always seen in traditional pharma led to improved results.
This article will look at enabling technologies, some of them already in action at existing and prototype facilities, that suggest drug manufacturing’s future. Enablers are combining in interesting ways that promise to allow greater use of continuous processing in the plants of the future. Peter Watler, CTO of Hyde Engineering + Consulting (San Francisco), notes, “A simple 45-cm-diameter chromatography column operating continuously could replace a complex and costly 200-cm column operating once per batch,” he says. “This will drive a need for enhanced unit operation science and superb automation, but it will result in reduced facility footprint, complexity and cost.”
They are also shrinking design-to-production timelines, which, Watler notes, have moved from 4-6 years a decade ago, to as little as 12 months and will soon shrink, for smaller facilities, to just six months. Eventually, he says, timelines will be dictated by shipping and assembly times.
At the same time, globalization has complicated the plant construction picture. Despite progress with harmonization, there is still some “discontinuity” of requirements from global regulators and, especially in more remote locations, there are questions of local suppliers and support, says Bikash Chatterjee, president of Pharmatech Associates (Hayward, Calif.), which is actively consulting in India and China. There is also a need, Chatterjee adds, to make information management a part of the plant design process from the very start. “We should take into consideration developing and applying IT infrastructure as part of facility design, assuming a global supply chain,” he says. In addition, he says, plant layout must be optimized early in the drug development process, to foster collaboration within the company and across cultures, which can be an issue for some Asian societies.
“Multicultural operations require collaboration,” he says. “In Asia, the focus is often on doing the opposite. Most offices and conference rooms have card key or key code access. Designing spaces that foster collaboration and integrate telecommunication technology that can bridge cultural differences requires thought.”
State of the art facilities in the future, Chatterjee says, will need:
• “social buildings” that foster interaction and team-based research and development
• open and closed laboratory designs
• flexibility to accommodate changes
• design for technology to provide access to electronic communication systems
• location in science parks to facilitate partnerships between government and the private sector
The Future? Modular and Plastic
For now, though, observers agree that many, if not most, of pharma’s new facilities will be modular. (See "Modular Construction in Pharma: No Longer a Novelty.") Despite the technology’s higher construction and transportation costs, manufacturers should continue to be attracted by the fact that it allows for smaller facility footprints and allows construction to progress in locations where cleanroom and piping expertise are hard to come by, says Hyde’s Watler. He foresees layouts becoming simpler and more standardized, driving costs down and enabling the construction of smaller, closed, single-use processing type plants in both developed and developing countries. He paraphrases Dr. Isaias Raw of Brazil's Instituto Butantan is saying that “developing countries no longer want to be ‘Coca-Cola bottlers,’ simply filling imported drug substance. They are moving to self-sufficiency for the therapeutics and vaccines unique to their regions, and small, modular facilities featuring single-use systems are well suited to meeting this need,” he says.
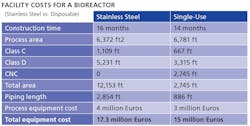
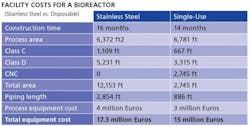
Working in synch with modular technology is disposable process equipment, which can help reduce investment and operating costs, and potential financial risk for anyone launching a new pharma product. Howard Levine, head of BioProcess Technology
Consultants, summarized some of the economic benefits recently at the World Vaccine Manufacturing Congress in France (Table).
Source: Levine, H., “Vaccine Manufacturing in the Coming Decade,” presented at the World Vaccine Manufacturing Congress, 2011, Lyon, France, October 11-12, 2011
Disposables can reduce labor costs by about 30%, Levine noted, although they result in 20% higher raw materials costs, offering a net savings, per manufacturing campaign, of about 10%, he said. However, additional savings come from the elimination of column packing and elastomer changeouts, as well as lower validation, calibration and equipment preparation requirements, as well as shorter processing time.
The leading edge of pharma’s new manufacturing base may be seen in vaccine manufacturing and niches such as personalized medicine, in such concepts as the pandemic-ready vaccine facilities being developed by Texas-based G-Con, LLC using its GMP-ready modular cleanroom technology. G-Con’s partners in various ventures include Xcellerex (Marlborough, Mass.) and GE Healthcare (Chalfont St. Giles, UK). GE is also collaborating with M+W Group (Stuttgart, Germany) offering turnkey construction solutions aimed at global markets.
Initiatives driven by the Gates Foundation, WHO and GAVI will spur development of more modular vaccine manufacturing facilities, allowing countries to tailor development to their unique regional needs, predicts Hyde’s Watler. One of the first such facilities was CPL Pharmaceuticals’ vaccine plant, a collaboration between Novavax (Rockville, Md.) and India’s Cadila Pharmaceuticals, which was completed in 2010 in Dholka, India.
In the U.S., increased government investment is stimulating more research and commercial activity in modular vaccine facilities using new plant, insect or animal cell culture platforms. Working with various plant cell culture platforms originally developed by Fraunhofer USA Center for Molecular Biotechnology are Project Greenvax, based in Texas, whose partners include Xcellerex, G-Con and Texas A&M University, and iBio, Inc. (Newark, Del), whose iBioLaunch platform was patented last October.
Hybrid Facilities: Finding the Right Mix
While mobile facilities would, by definition, be based on disposable equipment, it is unlikely that a large traditional type facility would be built with 100% disposable equipment. At larger scale, their cost and efficiency attractions diminish, says Watler. Most facilities on the ground today are opting for a hybrid approach, combining biodisposables with traditional stainless steel equipment. The challenge that manufacturers will have is determining the right ratio of each and where disposables can convey significant cost and operational advantages over traditional equipment.
Finding the right mix has been a challenge for DSM Biologics as it builds a major new facility in Brisbane, Australia. DSM uses the phrase "biologics plant of the future" to describe the project. The new site has a six-story shell in place that is being fit out in 2012. The second and third floors are empty for the time being, available for future build-out.
When it goes online some time in 2013, DSM Brisbane will become by far the largest biopharma contract manufacturer down under, says Ben Hughes, senior process engineer for DSM who is overseeing much of the project work. All upstream processes at the site, from media and buffer preparation through to bioreactors, are anticipated to be single-use, Hughes says. “We’ve shrunk the process suites by keeping all media and buffer concentrates outside of the controlled areas. The product and all solutions will be pumped through the walls into 500-liter bag-lined totes awaiting transfer or directly to the process equipment.”
“Downstream, we envision single-use for all of the filtration steps,” he says. “Chromatography will be more of a hybrid approach.”
“Single-use has all sorts of advantages in a CMO environment,” he continues. “It’s also really nice for us in terms of future-proofing the site. It’s extremely adaptable, with plug-and-play skids and so forth, so it’s easy to roll in new single-use technologies and roll out the older ones.”
It’s also about sustainability, Hughes notes, especially as single-use eliminates CIP and
SIP procedures and significantly reduces consumption of water, cleaning chemicals, energy and other resources.
Sense and Sustainability
The Brisbane facility is also a sign of the times in that it is the product of tight collaboration between DSM and the Queensland State Government and the Commonwealth of Australia, who provided financial assistance for the project. Around the time of the BIO annual meeting in 2009, BioPharmaceuticals Australia (BPA) requested expressions of interest to operate a major new bio contract manufacturing facility. It was fortuitous timing for DSM, says Hughes, as the manufacturer was looking to expand its biologics capabilities. DSM was selected as BPA’s partner and work began in 2010.
Section J of the Australian Building Code, enacted in 2010, calls for advanced greening of all new buildings, including, for example, significant increases in thermal insulation, window glazing and shading, rainwater collection, and high-efficiency equipment (e.g., HVAC and piped services with variable speed drive motors). A sophisticated Building Management System provides detailed operating and energy consumption data.
The facility will be oriented to the north for optimal solar design, is convenient to public transport, and has a large area for bicycle storage.
Any of these requirements alone is not groundbreaking, says Hughes. But together, they signal a new way of building drug sites—“heartening to see,” he says.
All of this comes in a smaller building than would have been imagined in the past—a mere 8,000 square meters. DSM’s proprietary technologies for productivity (XD for mammalian cell culture or Rhobust for expanded bed chromatography) will allow it to achieve in single-use bioreactors what would have required stainless steel bioreactors with 10 to 15 times greater capacity. “We’ve achieved a footprint that is much smaller than facilities built in the past but will still maintain the required output,” Hughes says.
Facilities of the future will be green, experts agree. According to estimates from the design and construction firm CRB, bulk biotech manufacturing facilities of the future can realize green savings in the neighborhood of:
• 75-95% water usage reduction
• 50-95% chemical reduction
• 50%+ energy reduction
• 25-50% smaller carbon footprint
• 30-40% smaller land requirement
When considering a hybrid installation, Hyde’s Watler says, the first question to ask is, is the single-use system designed from the ground up, or is it simply a modification of a conventional system? If it is simply a modification, did the workaround result in follow-on issues which must be addressed?
He uses the rectangular disposable bag in bin as a good example of a ground-up design. It is not a mere modification of a round stainless steel tank, Watler says, but rather, is rectangular, stackable, fits easily against a wall, has an open top and side panels for access—quite different from a conventional tank, and it works.
In contrast, buffer preparation tanks have been challenging. “There are no baffles so mixing is not as rapid, solids can settle in corners and crevices and it is a challenge to insert sensors into the tank,” Watler says. “The competition stainless steel mix tank, of proven design, with an optimized CIP cycle can be changed over in 15 minutes. At larger scales, this conventional tank may be the best option, operationally and cost wise.”
He recommends using FMEA and PHA risk analysis to sort through the mix of single use and conventional equipment. As facility design evolves and disposable systems become further incorporated, Watler says, there will be similarities between different sites, but not to the extent of standardization of hybrid facility designs.
What’s clear from talking to facility experts is that the drug manufacturing plants of the future are already here, being designed and built worldwide. They will only become more affordable, flexible, green, and commonplace in the coming years.