Single-use or disposable process equipment continues to be used in a wide range of biopharmaceutical applications, including upstream expression, filtration, purification, storage, mixing, processing and separations. Benefits include faster plant startup and changeovers, and less downtime. In the latest survey of biopharmaceutical manufacturing, which will be formally published in April, BioPlan looked at industry’s use of biodisposables in the manufacture of monoclonals, vaccines and other biopharmaceuticals. Here is a very quick look at only a few of the survey results.
Single-use or disposable process equipment continues to be used in a wide range of biopharma applications, including upstream expression, filtration, purification, storage, mixing, processing and separations.
Where Are Improvements Most Needed
This year, 352 respondents to the survey suggested that reducing cleaning requirements continues to be a key driver for moving to use of biodisposables, with over 48% of respondents describing it as “very important.” Other key motivators were decreasing cross-contamination risk, cited as “very important” by 42%; reducing startup times and capital investment requirements, cited by 40% of respondents; as well as reducing turnaround time and changeovers.
This year’s study suggests that the largest unmet need in single-use disposable equipment for bioprocessing is for lower cost single-use chromatography and ultrafiltration.
In addition, general industry consensus is that there is a continuing need for improvements in:
- Single-use sensors
- Larger bore single-use sterile connectors
- Better high-pressure applications
- Higher flow rate pumps
- Cost of commercially available connectors and tubing
- Capacity of single-use pumping
- Better device handling
- Manual or automated “valves”
- Process automation
- Better bioreactor design
- Applications for bacterial fermentations
- Standardization of materials and design
- Supply chain security
- Delivery, global material and specification standards
- Complete “start-to-finish” single-use products with scale-up possibilities
Responses also suggest that contract manufacturing organizations are using disposable process equipment at an even higher rate than biopharma operating companies. All CMO respondents said they were using disposable filter cartridges and tubing systems, for instance. For waste containers, 92% of CMO respondents were using biodisposables vs. 58.3% of biopharma operating companies, and for mixing 87.5% of CMO’s were using disposables vs. 50.4% of biopharma operating company respondents.
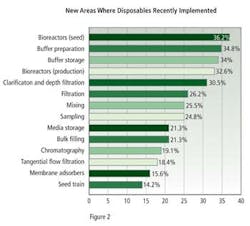
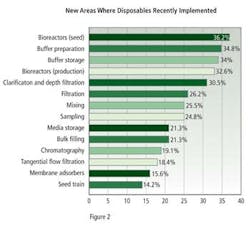
New applications for biodisposables, established within the past year, were dominated by seed and production bioreactors, with 36.2% and 32.6% of respondents describing this use, buffer preparation, with 34.8%, and buffer storage with 34.0% (Figure 2).
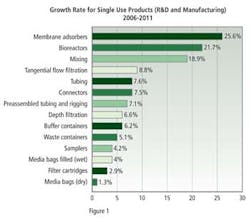
Respondents to this year’s survey gave a variety of reasons for not investing in biodisposable equipment: 13.6% noted that they had already made investments in existing equipment; 12.9% noted concerns about leachables and extractables; 10.6% noted the high cost of consumables; 9.8%, potential breakage of bags; 9.8%, that they had already validated existing systems; and 6.1% cited concerns over single sources and fear of potentially becoming dependent on a single vendor. Finally, 72.6% of respondents noted that they would increase their budget for disposable mixing equipment this year.
Reference
8th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, Preliminary Data, February 2011, Publication Date April 2011, BioPlan Associates, Inc.
About the Author
Eric S. Langer is president and managing partner at BioPlan Associates, Inc. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-scale Biopharmaceutical Manufacturing,” and many other industry reports. He can be reached at: [email protected], or at www.bioplanassociates.com.
| SURVEY METHODOLOGY: This eighth in the series of annual evaluations by BioPlan Associates, Inc. yields a composite view and trend analysis from 352 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 31 countries. The methodology also encompassed an additional 186 direct suppliers of materials, services and equipment to this industry. This year’s survey covers such issues as: new product needs, facility budget changes, current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, and employment. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and CMOs. It also evaluates trends over time, and assesses differences in the world’s major markets in the U.S. and Europe. |