As Biopharma Matures: Working Harder, Getting Leaner
Biopharmaceutical manufacturing has not been immune to the "do more with less" mantra driving all industries for the past two years. Reflecting squeezed budgets and the mixed signals of a gradual economic recovery, biopharmaceutical manufacturers are spending more on new technology adoption, training, capital equipment and efficiency improvements, but less on hiring, outsourcing and new facility construction [1].
As the industry matures, it has become better able to respond to acute problems such as capacity crunches. The question now is how well biopharmaceutical manufacturers can address chronic issues that have been growing in importance over the past seven years, including the costs of downstream operations, the need for real-time process monitoring, and issues entailed by disposing of disposables.
This year, 327 global biomanufacturers responded to BioPlan's Seventh Annual Report and Survey of Biomanufacturing. Data suggest that they are more focused on improving manufacturing performance using current resources: improved process development, better process control, more effective use of disposables, and more-efficient processing, both upstream and downstream.
Chronic Problems in 2010
Nagging problems tend to be those less easily resolved, requiring technological advances or regulatory interventions, and more time for the industry to adopt and absorb. Among the major chronic problems this year's survey identified include:- Downstream Processing & Chromatography Steps: The costs and staffing requirements for managing downstream processing operations, especially chromatography. Companies also realize that fixing these operational areas will likely require time and technology. For example, in downstream processing, while 42% indicate it is the factor most likely to create capacity constraints over the next five years, only a small percentage in previous studies (9%) were able to identify where those fixes might come from.
- Implementation of Quality Management: Implementation of Quality by Design (QbD) and Process Analytical Technology (PAT) programs has been slow. While two-thirds have the budget for QbD initiatives, over 50% simply don’t have the staff available to implement programs. If implementation were a more acute pain, then it is likely that staff would be prioritized to address the issues.
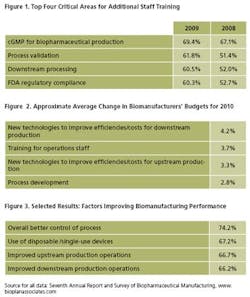
- Training Budgets: Nearly 70% of respondents this year indicated that additional staff training is a “High” or “Very High” need within in GMP areas; and 62% saw training in validation as a priority (Figure 1). This year's training budgets are up by nearly 4% (Figure 2). Yet, the total amount of training per employee is declining. For example, we asked how much training manufacturing operations staff receive per year, and the number has decreased: 39.4% received more than 11 days of training in the current survey, compared with 44.6% last year.
- Process Control: Of the 15 areas we measured, the number one factor this year that led to the greatest improvements in biomanufacturing has been implementation of better process control. This was indicated by 74% of respondents (See Figure 3). Manufacturers and their vendors are likely to continue putting resources toward process improvements as costs of manufacturing rise, and hiring budgets shrink.
Layoffs Create Acute Problems
The acute problems this year center on the fallout from the layoffs that have occurred the past couple years. Companies are analyzing their operations, and retraining or hiring for higher value-added activities. For example, process development budgets are up nearly 3% this year (Figure 2), and the biggest growth is in technologies to improve downstream operations. This is not surprising, since it is where the majority of biomanufacturers indicate they are experiencing the greatest challenges. It is also one of the few hiring areas that are increasing in 2010.
Hiring is, of course, tied to changes in outsourcing. Despite layoffs, the work requirements don’t go away. A question companies are now asking more frequently is what work will be done in-house and what will be outsourced. Internal restructuring and outsourcing needs to be done, and staffing and hiring problems in areas like process development and downstream operations have led to reallocation of internal staff. Paul Mehelic, PhD, principal scientist and group leader for Global Biologics at Pfizer, who spoke at an IBC meeting in Carlsbad, California in March, noted that Pfizer’s outsourcing approach involves determining strategically what the company can “outsource [to] reduce costs, optimize speed, increase flexibility of our internal operations . . . redeploy internal FTE’s on more challenging, and more value-added activities.” Outsourcing decisions are based on analysis of the value of full-time employee activities.
Hiring budgets for scientific staff, for example, are down 1.3% this year, and new operations staff hiring budgets are, at best, neutral. However, people are being hired in areas involving process improvement, where perceived value can outweigh the costs of new hires.
Operational Changes in 2010
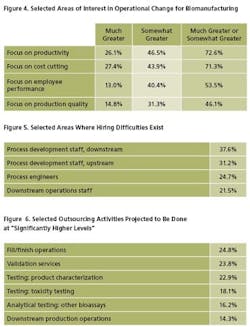
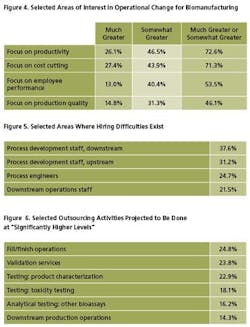
In addition to productivity, companies are targeting cost-cutting, employee performance, and production quality. Interestingly, this year, only 15% indicate they are focusing on “production quality” to a “much greater” extent. This suggests companies may be targeting productivity, possibly to the detriment of quality, as they are forced to do more with less.
Changes may also involve fundamental shifts is process technologies to boost productivity. According to David Onions, PhD, CSO at BioReliance, “Traditional platforms for protein production will meet a barrier . . . [where] we will have to turn to engineering ‘professional protein exporting cells,’ perhaps from stem cells, or non-mammalian systems engineered with human glycolsylation pathways.”
Hiring in Manufacturing Areas
With the recent, massive layoffs in the industry, hiring in 2010 will continue to be a problem. In specific areas, however, especially where efficiencies and process improvements are required, demand for new hires is up. This is likely fallout from the budget crunches, as companies look toward process improvements to fill the gaps left open by hiring freezes and layoffs.
The industry's top hiring challenges this year include “Process Development, Downstream,” where 37.6% of respondents indicate they are currently having trouble filling positions (Figure 5). Following are “Process Development Staff, Upstream” (indicated by 31.2%), and Process Engineers (24.7%). Downstream operations, not surprisingly, also figure prominently, with more than 1 in 5 companies indicating that they can’t fill positions. Regulatory staff are also in relatively high demand.
Outsourcing Drivers
Biopharma companies plan to do more outsourcing than in the past, survey data suggest. From the study responses, critical decision factors are not cost-driven, but rather are focused on factors such as quality, and establishing good working relationships. Most biopharmaceutical companies have turned to outsourcing to control costs and manage their internal staff and resources so in-house activities are done cost efficiently. Economic conditions today are causing organizations to re-evaluate their core competencies and decide how they will direct their R&D and manufacturing resources. Outsourced projects are now used not only to fill temporary gaps in capacity, but also to focus a company’s skill base on core competencies, in addition to controlling costs.
This growth is especially true for current activities typically outsourced. In our study, we tested 24 different areas of outsourcing today. We found, for example, that 25% of respondents to the study will be outsourcing significantly more fill/finish operations over the next 24 months than is currently done (Figure 6). Following are validation services and product characterization testing (24% and 23% of respondents, respectively). Other areas of substantial growth include toxicity testing and downstream production operations (where 18% and 14% of facilities, respectively, will see substantial changes).
|
Industry Trends Exacerbating Problems
|
Given the more rigorous regulatory standards being imposed on manufacturers, it was interesting to note that relatively few respondents expected areas such as GMP training to be outsourced. Presumably, companies wish to keep tight control over specific aspects of operations and management. Biologics manufacturers may not yet be sufficiently confident that their outsourcing partners can guarantee quality standards.
Outsourcing today continues to be dominated by relatively lower value-added services, such as fill-finish and product characterization testing. We tested 24 areas of outsourcing in the study, and found that the primary outsourced activity today, with over 75% of biopharmaceutical companies outsourcing at least some of this activity, was product characterization testing.
Summary of Trends and Problems in 2010
Overall, 2010 will be a good year for the biotechnology and biopharmaceutical industries. With traditional R&D approaches failing to live up to expectations in terms of producing new drugs, many have looked to biotechnology to reinvigorate drug development and provide recognizable medical advances.
BioPlan's Annual Report demonstrates that process improvement and outsourcing are now taking on greater importance, with most companies realizing the strategic necessity to more efficiently use internal resources, and focus on core competencies. These changes will likely require time, as current economic, hiring, and budgetary realities settle in. All areas of R&D and manufacturing are now considered options for outsourcing and the impact of this is being felt on a global basis, with emerging markets ranking favorably alongside established markets as outsourcing destinations.
Most companies are increasingly confident, but are also more realistic: 2010 will bring good results, this year's survey suggests, but managers are expecting greater productivity and better performance from the same staff. Companies are finding the need to refocus their efforts on delivering productivity and streamlining operations, while maintaining high quality standards. This lean strategy, consistent with virtually all other manufacturing-oriented industries, is a prerequisite for solid future growth. And as in other industries, with lean manufacturing comes the question of how to ensure product quality.
| Survey Methodology: This seventh in the series of annual evaluations by BioPlan Associates, Inc. yields a composite view and trend analysis from 327 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) in 35 countries. The methodology also encompassed an additional 125 direct suppliers of materials, services and equipment to this industry. This year's survey covers such issues as: current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, employment and training. The quantitative trend analysis provides details and comparisons of production by biotherapeutic develops and CMOs. It also evaluates trends over time, and assesses differences in the world's major markets in the U.S. and Europe. |
References
1. Seventh Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates, Inc, Rockville, MD. April 2010, www.bioplanassociates.com.
2. Biopharmaceutical Products in U.S. and European Markets, Seventh Gen Web, available: www.bioplanassociates.com.