Most of us in the biotechnology industry recognize that it is very investment-dependent. Yet even with this knowledge, many of us have been shocked at how rapidly the environment has changed with the current financial crisis. Biotherapeutic manufacturers and industrial biotechnology companies, of course, are affected by the capital markets. But now, vendors and service suppliers are feeling the pinch. Though the healthcare industry has historically been able to weather economic downshifts, the segment’s short-term path in 2009 is likely to be shaped more by events on Wall Street than by prospects for new technologies or product approvals.
Biotech trade groups and analysts describe the impact of the global financing crisis in no uncertain terms: initial public offerings (IPOs) have fallen to virtually zero this year, and a recent Biotechnology Industry Organization (BIO) report showed that 30% of publicly traded biopharmaceutical companies have less than six months cash on hand. Though private funding levels remain fairly consistent with previous years, analysts are predicting a tough go in 2009 for both public and private companies. More than 6,000 biopharma employees have been laid off from over 100 companies since the crisis began. Mergers and acquisitions may accelerate these shifts: for example, Merck and Schering-Plough plan to cut 16,000 pharma jobs, post-merger.
Despite all this, some of these shifts will open opportunities. For example, outsourcing activities are increasing as companies deal with internal costs. This is an ongoing trend in manufacturing, R&D, clinical trials, and now, even sales outsourcing is on the rise. Pfizer, the largest pharmaceutical company, reportedly plans to lay off up to a third of its reps and go with contractor sales. While such actions may improve short-term results, they are often done at the expense of investor equity in the long term.
In my company’s annual industry report, the 6th Annual Report and Survey of Biopharmaceutical Manufacturing [1], we evaluate trends and the extent to which global manufacturers and vendors are being squeezed. Budget projections are an early indicator and our study attempts to quantify how these fears may translate into budget contractions. The results provide a global view from executives at 446 biopharmaceutical manufacturers and contract manufacturing organizations.
Global Biotechnology Trends
The obvious financial upheavals are taking attention from some of the important trends we’re seeing in the industry. Some of these will become opportunities as we pass through the current financial crisis:
- Product approval trends
- Regulatory shifts and biosimilars
- Trends and opportunities in emerging markets — China, India, Middle East
- Scientific trends: expression systems, biofuels, stem cells
- Manufacturing trends: disposables, downstream, outsourcing trends
Product Approvals on the Way Up
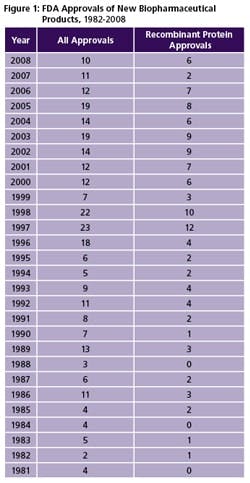
(Click to enlarge image) Figure 1: FDA Approvals of New Biopharmaceutical Products, 1982-2008
Primary Approval Trends: There has been a general downward trend in biopharma product approvals since 2005 (see Box at end of article), especially for recombinant proteins and monoclonal antibodies. However, we believe this is likely to change over the next five years [2]. Between 1996 and 2005 there was an average of 16.6 approvals per year, compared to only 10 new product approvals in 2008 (Figure 1). Trend-wise, these 10 new products involved a greater percentage of recombinant proteins, with higher peak sales levels. Further, few monoclonal antibodies and cancer therapeutics have been approved in recent years, despite many reports about large numbers in late development. On the bright side, there were a large number (more than 25) of filings currently pending, and an equally large number are expected to be filed in 2009. So there should be an increase in approvals in 2009 and the next few years.
Biosimilars: The advancements in biosimilars are a long-term trend that will continue to be fueled by improved manufacturing technologies, cost constraints, and political and healthcare policy issues. The European Union has formally adopted regulations for their approval and has approved multiple biosimilars, including versions of erythropoietin (EPO). Several bills that will enable FDA approval of biosimilars have already been reintroduced in Congress in 2009. This will formally enable FDA to grant generic drug-like approvals for most biopharmaceuticals.
Merger and Acquisition Trends
The consensus of opinion from analysts in the industry is that both large and small biotechs are likely to be scarred by this economic period. Some will find the only way out is to be acquired. Others will restructure or sell off valuable assets. In this cash-tight environment, those without deep pockets are likely to find it a very difficult year. In particular, smaller public companies are at risk for dramatic and rapid reversals. IPOs have virtually disappeared, and venture-backed life sciences companies are finding it next to impossible to expand, or develop their technologies, as access to capital dries up. The ripples from this upheaval are likely to continue for some time. Although private sources of funding continue to be available (current funding of private sources is more or less on par with previous years), how private deals will be structured for small biopharmas surviving the financial pinch will likely create a lasting effect on the industry. This trend will continue as larger pharmaceutical companies begin to acquire rights to market more products from smaller, struggling companies. Here, we are likely to see more deals cut for bargain-priced, top-quality technologies.
Emerging Market Trends
China Opportunities: The current global economic situation has pushed some of China’s key manufacturing indexes to record lows; yet Western economists are still projecting positive annual growth rate for China in 2009. Some of this growth will come from investments and growth in China’s biopharmaceutical sector. Recent collaborations, and investments in China’s biotech industry, represent a sustainable growth trend, despite retreating global investments. According to Bioplan Associates’ recent analysis of the biopharmaceutical manufacturing sector, Directory of Top 60 Biopharmaceutical Manufacturing Organizations in China [3], investment and growth in this emerging industry segment will outstrip both global and Chinese manufacturing sector expansion. Chinese domestic growth is fueled by joint ventures and R&D collaborations between Western and Chinese organizations:
- GlaxoSmithKline (GSK) signed an exclusive agreement with Neptunus Interlong Bio-Technique Co. Ltd. (NIBT) for the co-development of influenza vaccines.
- Novo Nordisk announced plans to invest $400 million for an insulin manufacturing facility in Tianjin.
- Sanofi-Aventis announced plans to collaborate with the Chinese Academy of Science on cancer-related stem cell research.
- Genzyme Corp. plans to build an R&D center in Beijing as part of its continued global expansion, to open in 2010, with project costs reaching $90 million.
In addition, we are seeing growth trends including:
- Expanding national health insurance coverage to make healthcare more accessible to its 1.3 billion population.
- Bigger domestic biological consumption as China’s industry catches up in the R&D and process development side of biologicals. Dozens of products (mostly biogenerics) developed by Chinese researchers have made their way into Chinese markets. The next drugs in the pipeline are Chinese-innovated.
- R&D bottlenecks increasing Western collaborations: China is now home to more than 580 biopharma companies (although over 500 are small enterprises of less than $10 million) [4]. Making investment in R&D is extremely difficult in this economic climate. Thus, in the foreseeable future, the Chinese government is likely to continue to invest heavily into biotech research at universities. China has plenty of western-educated biotech talent. However, commercialization of biotech innovation, process development, and manufacturing are often decades behind mainstream markets.
- Biotech exports—biogenerics: Over the next 3 to 5 years, Chinese biogeneric makers will test the waters in developing countries including those in the Middle East and Asia.
- Bio-reagent exports: Exports of bio regents will increase, as China has been exporting bio reagents and research-use antibodies for years. The economic recession may actually benefit Chinese reagent makers as manufacturing costs are cheaper in China, and quality appears to be comparable.
India Opportunities: India’s biopharma sector continues to expand, even with the current economic slowdown. Revenue in the sector in 2008 exceeded $2.5 billion, with an estimated annual growth rate of 25 percent [5], although this is likely overestimated in today’s economic climate. The year 2009 will continue to find over half of Indian biopharma products and services sold abroad as exports. In addition, Indian basic R&D, clinical research and production capacities have continued to expand to support the sector’s growth. This growth has not been without glitches. Recent events can be projected to future trends in the region:
- New regulatory authority for biopharma nears startup: Indian officials plan to elevate the Department of Biotechnology to full ministry status.
- R&D expansions: Biopharma multinationals are offshoring increasingly large slices of R&D, as they find international firms with the technical capabilities they require. India, in competition with China, Singapore, Korea and other fast-growth nations, aims to capture as much of that market as it can. Major new R&D facilities were announced for Avesthagen (in Bangalore), Panacea Biotec (Mumbai) and Biocon (Punjab); and biotech parks with R&D facilities, such as Bangalore Helix and the Genome Valley expansion, continue to proliferate.
- Clinical research: The clinical research market in the country for all drugs, large- and small-molecule, is growing, from ~$300 million in 2008. So far, however, India has been unable to exploit this market opportunity as fully as its CRO industry would like, due to problems of bureaucratic complexity, and lengthy delays for trial approvals.
- Substandard Indian vaccine producers shut down under WHO pressure: Indian government inaction reportedly caused WHO to threaten a suspension of purchases for its global vaccination campaigns.
- IP climate complaints continue: U.S.-based BIO organization publicly demanded that the Office of the U.S. Trade Representative keep India (and other countries) on the USTR’s “Priority Watch List”. Regulatory officials in India have been considering requiring biopharma developers to obtain product patents even for “biosimilars” due to the complexity of the large-molecule evaluation process.
India’s biopharma industry is managing to do quite well on the strength of contract research plus local and regional sales of insulins and other off-patent, big-market biologics. The current economic downturn in North America and Europe is likely to hasten the transfer of basic R&D, clinical research and manufacturing activities to India and other low-cost, high-tech countries. Meanwhile, Indian-made biogenerics are competing in the Indian domestic market and are increasingly being sold in other lightly regulated markets in the Middle East, Africa and Asia. Now open to biogenerics, Europe is the next big target, and legislation to enable biogenerics approvals in the U.S. is advancing, as an emphasis on healthcare cost cutting is expected. Further into the future lies growth driven by innovative drugs, vaccine initiatives, bioinformatics expertise and clinical trials prominence.
Science Trends
Expression Systems. A major trend area in biopharmaceutical manufacturing involves the use of novel expression systems. Based on our research [1], nearly 50% of biomanufacturers today are demanding more from their current primary expression systems. Manufacturers are balancing tried-and-true classic expression systems, and their relative regulatory safety, against the opportunities for significantly higher yields available from newer platform technologies [6]. Nearly all current products are being produced using E. coli, Chinese Hamster Ovary (CHO) cells and the yeast Saccharomyces cerevisiae (S. cerevisiae) as hosts. Many new platforms exist featuring new host cells and organisms. Of course, regulatory factors play a major role here, as do issues surrounding intellectual property (but even here, as many as 46% of biomanufacturers have indicated they would be willing to pay royalties for improved yield) [1].
We explored the potential for adoption of new expression systems and were not surprised to find that 55% of respondents at biomanufacturing facilities indicated they would consider an alternative expression system in early R&D. We also found that 43% of respondents in process development stages would consider alternative expression systems. This suggests that for new drug products, expression system technologies are not written in stone.
Biofuels Trends
Survey MethodologyThis sixth in the series of annual evaluations by BioPlan Associates, Inc. yields a composite view and trend analysis from 446 responsible individuals at biopharmaceutical manufacturers and contract manufacturing organizations in 35 countries. The methodology also encompassed an additional 140 direct suppliers of materials, services and equipment to this industry. This year’s survey covers issues such as: current capacity, future capacity constraints, expansions, use of disposables, trends and budgets in disposables, trends in downstream purification, quality management and control, hiring issues, employment and training. The quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and contract manufacturing organizations (CMOs). It also evaluates trends over time, and assesses differences in the world’s major markets in the US and Europe. |
Biofuels continue to be a major industrial biotechnology trend. Corn ethanol demand remains strong despite the collapse of oil prices. In 2008, the U.S. ethanol industry produced and sold a record nine billion gallons, and capacity grew by 34% (with more than 30 new facilities opening, so total capacity is now more than 13 billion gallons) [7]. However, according to David Mousdale, CEO at beòcarta Ltd (London), a specialist biotech consultancy active in biofuels, and co-author of our new book on biofuels, “the collapse of the credit markets has prevented second-generation technologies from securing financing to accelerate the commercialization of cellulosic ethanol from nonfood crop sources. Despite this, Royal Dutch Shell announced in March 2009 that it will no longer invest in renewable technologies such as wind, solar and hydro power because they are not economical; instead, it plans to invest more in biofuels.” Additionally, the Japanese have a solution to the food-versus-fuel dilemma: “high-biomass sugarcane” to create ethanol without sacrificing sugar output. Other biofuels are being found to be more economically feasible, including microbial biohydrogen alcohols.
Manufacturing Trends
Bioreactors: The effective size of bioreactors is likely to go down over the next five years. As titres improve and upstream production efficiencies continue to generate higher product yields, the need for the additional capacity is decreasing concurrently. In our 6th Annual Biopharmaceutical Report, we found that the top trend toward acceptance of these products continued to be regulatory acceptance of data for leachables and extractables (indicated by 82% as “Important” or “Very Important”). Other critical factors were issues of quality control records, and engineering data for mass transfer, mixing, shear, etc.
Disposables: The increased use of disposables in biopharmaceutical manufacturing is an ongoing trend. Bags, bioreactors, sampling systems, mixing units, and other applications all add to the flexibility of a facility, which is especially important to CMOs where multi-campaign equipment requires rapid changeover times, faster cleaning, and lower risks of contamination. The reach of disposables will extend even further over the next five years, as companies switch from stainless steel tanks. This trend is driven by the need to reduce capital and cleaning costs.
The rate of usage of disposables in biopharmaceutical manufacturing increased substantially from 2005 to 2008 in a number of disposable areas.
- Use of membrane adsorbers jumped from 12.9% to 49% of respondents reporting using these devices (a compound annual growth rate, CAGR, of 39.9%).
- Mixing systems usage grew to 55.6% in 2008 (a CAGR of 30%).
- Bioreactor usage grew from 21% in 2005 to 60.6% in 2008 (a CAGR of 30%).
When asked to indicate the most critical reason for using disposable technologies, this year, the number one reason was to “Reduce Capital Investment”, indicated by 14.4% of respondents (and by 78% of participants as being “Important or Very Important”). The attribute, “Eliminating cleaning requirements” (“indicated as “Important” or “Very Important” by over 88% of participants) was noted by 13.1% as being their “Most Important” reason. This could be a function of where respondents were regarding stages of facility expansion or construction. To summarize the top four “Critical Reasons”:
- Reduce capital investment in facility and equipment
- Eliminate cleaning requirements
- Decrease risk of product cross-contamination
- Faster campaign turnaround
Downstream Purification
The big trend over the past few years has been for improved downstream purification and separation technologies. The costs associated with downstream purification can run up to 40% of biopharmaceutical manufacturing costs. Today, as upstream titres increase, pressure is building to find more efficient and cost-effective purification systems. In our study, the impact that downstream purification has on capacity is seen as the largest issue, with 54% indicating it is at least a “significant bottleneck” to production capacity. Other growing downstream trends are for alternatives to Protein A. In this year’s study, 59% of biomanufacturers indicated they “Agreed” or “Strongly Agreed” ; that they were considering alternatives to Protein A to reduce costs for new production units.
Increased Outsourcing for Biopharmaceuticals
Biopharmaceutical product developers are reinforcing their internal focus on research and development, discovery, and marketing while strategically outsourcing certain manufacturing and process development activities. This will continue to feed the growing demand for CMO manufacturing capacity. Drug manufacturers will increasingly consider contract manufacturing more as an asset to drive strategic manufacturing decisions than as a simple capacity alternative. In the future, we are likely to see a continued, rapid maturing in this industry, and increased arrangements that stress sharing of risks, and developing partnering agreements between CMOs and biopharmaceutical product developers.
Most CMOs today have developed very efficient and compliant biomanufacturing capabilities, and outsourcing has become standard practice. Small biotech companies have fewer options and move toward outsourcing more readily. Large pharmaceutical companies with limited expertise in biomanufacturing process development may opt for outsourcing. And those with experience and capacity may still need the flexibility a CMO affords to handle overflow capacity.
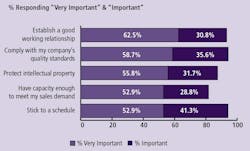
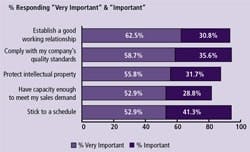
(Click to enlarge image) Figure 2: Critical Issues when Considering Outsourcing Biopharmaceutical Manufacturing to a Contract Manufacturing Organization (CMO), 2008
So the trend appears to be not so much whether to outsource, but rather what, and how much to outsource. Today, only 52% of biomanufacturers do all their manufacturing for mammalian cell culture in-house (the ratio is 60% for microbial fermentation). By 2013, the percent of biomanufacturers not outsourcing will decrease to 47% for mammalian cell culture.
Reasons companies will be increasing their outsourcing to CMOs will be dependent on how well CMOs work with their clients. The trends over the past six years in which we have measured these factors have not changed significantly. “Establishing a good working relationship” has consistently held near the top of the list, along with soft attributes such as being able to stick to a schedule (Figure 2).
Other Future Trends
Offshore Production and Development: While this can lower labor costs, it can significantly increase project management, general management, rejection rates, logistics, and distribution costs. Unless done right, total costs can sometimes equal or exceed the savings from the lower labor costs. Currently, the trends for the top outsourcing destinations outside respondents’ home countries are to: U.S., Germany, and the UK (indicated as a “likelihood” or “strong-likelihood” by 36.8%, 21.3%, and 13.5%, respectively).
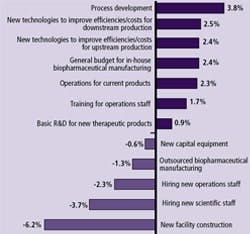
(Click to enlarge image) Figure 3: Avg. Change 2009 Budget: BioManufactureres & CMOs
Capacity Utilization Trends: Overall capacity utilization by biopharmaceutical developers and contract manufacturers has leveled out, due to stabilized industry expansion and improvements in yield at existing facilities. Capacity utilization for all biomanufacturers using mammalian cell culture systems has been very stable since 2003, and is currently at 63.3%. (In 2006, it was 63.9%.) Capacity utilization for microbial fermentation is 55.3%.
Budget Trends
Budget projections are an early indicator of financial strategy. In our annual biomanufacturing study [1], we attempt to quantify how fears from today’s current financial environment may translate to budget shifts. The results, from a global view, show how executives at 446 biopharmaceutical manufacturers and CMOs will be changing budgets and buying patterns.
Figure 3 shows that respondents (as recently as January 2009) are indicating most budget areas are likely to remain unchanged over the next 12 months — good news for departments, and their vendors. The bad news, for some vendors at least, is that these budgets are going to be spent more carefully.
Biomanufacturers’ Budget Trends: Areas of significant change in biopharma companies’ budgets included process development, where the greatest budget increases are likely (3.8% on average). Following is budget increases for new technologies to improve efficiencies for downstream production (2.5%). These shifts mirror general trends toward more upstream and downstream productivity in biomanufacturing. Despite the leaner purchasing environment, downstream areas are still being funded. However, many are being careful to remove less critical elements from RFPs. This sometimes means delaying equipment purchases, doing more thorough analysis on the cost implications of acquisition, or negotiating harder with vendors. Not unexpectedly, the areas of greatest budgetary decrease are in new facility construction (down 6.2%). Interestingly, the change in budgets for new capital equipment appears to be relatively flat (-0.6%).
Vendors’ Budgetary Trends: Vendors have also found that biopharmaceutical clients continue to have budgets for projects, but are being more deliberate in their decision-making. Today, decisions are requiring more substantial financial analysis to support implementation. The need to improve their sales calls may be a reason that vendors’ only budgetary increase this year is going to be to their sales staff. In fact, vendors’ experience may be a bellwether for industry growth as a whole. Decision-makers’ cautious buying may result in delayed purchasing, and a wait-and-see attitude among buyers.
(Click to enlarge image) Figure 4: All Segments Sales Growth: Vendor Sales to BioManufacturers
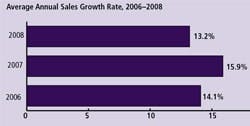
Vendors’ Annual Sales Growth: To evaluate the impact of the current economic situation on vendors to this industry, this year we also queried 140 vendors regarding their average annual growth rates. This establishes a “derived demand” for products used by the biopharmaceutical industry, which in turn provides insight into the growth rate of end users of these products. In our study we found relatively robust growth for 2008: on average, vendors reported an annual growth rate of 13.2% (Figure 4). Whether this is sustainable in 2009 is problematic.
Summary
Despite the current economic situation, and caution among decision-makers to commit to projects, biotechs continue to secure private capital, and merger and acquisition activities will continue to ensure that good science opportunities and investments continue to flow. However, earlier-stage companies will likely need more compelling science to attract necessary capital in the short term. Some analysts are recommending that startup life-sciences companies look overseas for VCs, investors, partners, and capital. Perhaps some of this investment may come from the Middle East [8] and Asia.
About the Author
Eric S. Langer is president at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, Md. in 1989. He is editor of “Advances in Large-scale Biopharmaceutical Manufacturing, 2nd Ed”, and publisher of “Biopharmaceutical Expression Systems: Current and Future Manufacturing Platforms”. He can be contacted at [email protected].
References
1. BioPlan Associates. 6th Annual Report and Survey of Biopharmaceutical Manufacturing and Scale-up Production. April 2009.
2. Rader, Ronald A. Biopharmaceutical Products in the U.S. and European Markets: http://www.bioplanassociates.com/biopharma.com.
3. Zhou, Y.B. Directory of Top 60 Biopharmaceutical Manufacturing Organizations in China. BioPlan Associates, Inc. April 2008.
4. BioPlan Associates, Inc. Advances in Biopharmaceutical Technology in China, 2007.
5. BioPlan Associates, Inc. Advances in Biopharmaceutical Technology in India, 2008.
6. Rader, Ronald A., Biopharmaceutical Expression Systems: Current and Future Manufacturing Platforms. BioPlan Associates, Inc. November 2008.
7. Mousdale, D., Drumm, L. Quick Guide to Biofuels: For when the oil runs out. BioPlan Associates, Inc. Publication date est. June 2009.
8. Kermani, F. Quick Guide to Biotechnology and Healthcare in the Middle East. April 2009.
FDA Approval TrendsFDA approvals of biopharmaceutical products have decreased significantly in recent years [2]. This noticeable decrease includes recombinant proteins and monoclonal antibodies and cancer therapeutics. In the decade 1996-2005, there was an average of 16.6 approvals per year, while there were only 12, 11 and 10 new product approvals in 2006, 2007 and 2008 respectively. The 10 new products in 2008 is the lowest number of approvals of new biopharmaceuticals since 1999. Among the encouraging findings for 2008, relative to 2007: a) there was a major increase in the number of recombinant proteins; b) 2008 new products are expected to attain significantly higher peak sales levels; and c) more products came from smaller U.S. biotechs. None of the 2008 products are expected to attain peak blockbuster (>$1 billion/year) sales, unless approved for new indications. The only 2008 approvals for truly new indications (diseases) were two products approved for orphan (rare) diseases. Two 2008 products, which many might now consider to be follow-on proteins or biosimilars, received abbreviated 505(b)(2) generic drug approvals. Hardly any monoclonal antibodies and cancer therapeutics have been approved in recent years, despite many reports (much hype) about large numbers in late development and claims that these products are revolutionizing patient care. Only one MAb product received approval in 2008, 2007 and 2006. There is no consensus regarding why FDA approvals of biopharmaceuticals, and also all pharmaceuticals, have decreased in recent years. The decline in approvals is often attributed to unnecessary delays and denials by FDA. For example, a number of biopharmaceuticals have received “approvable letters,” and multiple product evaluations have been delayed or their applications denied. In the context of Vioxx and other drug safety-related problems and controversies in recent years, many point to FDA having become more conservative, more risk-averse in its product approvals. Also, FDA may simply not have sufficient staff to complete the work expected of it. Others cite industry as all too often conducting inadequate clinical trials, cutting corners and otherwise rushing product development. However, it is encouraging to note that few filings for biopharmaceuticals have been denied, delayed or put on long-term hold without obvious reason. Problems often relate to trial protocols or attaining primary endpoints in pivotal trials. The low number of recent biopharmaceutical approvals is not due to fewer products in development. Pipeline databases and other sources generally show that the number and percentage of biopharmaceuticals vs. other pharmaceuticals have been steadily increasing. Rather, it appears that fewer products are making it through pivotal Phase III trials to final approval stages. Newer technologies for design of antibody-like molecules are finally being reflected in approved products. In 2008, two engineered MAb-like molecules received approval. However, the long-term trend of recombinant proteins and antibodies being manufactured using the same old, largely 1980s, manufacturing technologies (e.g., E. coli, yeast and CHO expression systems) continued. All of the recombinant products approved in 2008 involved use of these now-aging expression systems. Despite 2008 continuing the trend of a low number of approvals in recent years, the year will almost certainly be a recent low point, with biopharmaceutical approvals expected to steadily increase in coming years. A large number (more than 25) filings are currently pending and an equally large number are expected to be filed in 2009, so there should be an increase in approvals in 2009 and the next few years. These filings include a number having previously experiencing setbacks/problems (presumably fixed) with their clinical trials and other parts of their applications. Besides a large number of products either pending or with filings expected, mostly recombinant proteins and monoclonal antibodies, in coming years there will be new classes of products. These include biosimilars/follow-on proteins, of which there will be a large number, and many products long in development, some for decades, including gene therapies, stem and other cellular therapies, cancer vaccines and individualized therapies, along with newer technologies such as RNAi. Many biosimilars, despite containing much the same or similar active agents as prior products, will be substantially innovative and will likely offer improvements, such as novel methods for administration and will likely be marketed as innovative rather than true generic, knock-off products. |
Biosimilar Products AdvanceAnother major trend in biopharmaceuticals, one that will affect the entire industry, is the advent of biosimilars (or follow-on biologics, biogenerics). This is simply a by-product of the biopharmaceutical industry maturing. Patents are expiring for many products, including those developed in the first two decades (1970s/’80s) of recombinant proteins and monoclonal antibodies. Biosimilars are already a fact in Europe, with the European Union having formally adopted regulations for their approval and having approved (and also denied) multiple biosimilars, including multiple versions of erythropoietin (EPO). Biosimilars or follow-on biologics (FOBs) involve biopharmaceuticals with their active agents and often final formulations similar in many respects to one or more prior biopharmaceuticals (regulated as biologics). Generally, biosimilars will be off-patent variations or "me, too"-type versions of previously approved biopharmaceuticals, with this similarity presumed to facilitate regulatory approval of these products based on comparisons with the well-established, similar reference product. To date, terminology and definitions related to these products have little consensus. Several bills that will enable FDA approval of biosimilars have already been reintroduced in Congress in 2009. One of these, or more likely a cobbled-together compromise, is expected to be enacted this session. This will formally enable FDA to grant generic drug-like approvals for most biopharmaceuticals—i.e., those regulated as biologics (while those fewer, simpler biopharmaceuticals regulated as drugs already have generic approval pathways, and there are multiple such generic biopharmaceutical approvals). Like generic small-molecule drugs, these biological product approvals would be largely based on analogies and comparisons with a previously-approved reference product. Biosimilar applications will be abbreviated in the sense that they will largely be based on biochemical and bioequivalence trial comparisons with the reference product, usually with no large (expensive and time-consuming) placebo-controlled Phase III trials. Major issues related to biosimilars will need to be resolved by Congress and interpreted by FDA. The most ardently-contested aspects of biosimilar legislation include data exclusivity and the extent to which (or not) regulations will follow long-established patterns for generic drugs originally specified in the Hatch-Waxman Act, such as use of generic or nonproprietary names. Data exclusivity involves FDA not allowing a biosimilar to be approved based on comparisons with data from another previously-approved reference/comparator product. This is not market exclusivity, as is granted by orphan designation, since these same products could be approved, if full BLAs are filed. The main issue in contention is not whether data exclusivity is needed to provide some minimal protection for innovative products from biosimilar competition, with even biosimilar interests recognizing the value of this. Rather, the issue is how many years of data exclusivity to grant to established products. As can be expected, biosimilar interests favor fewer years — e.g., five or less — and established innovator companies favor more — e.g., 12-14 years. Another major biosimilar issue to be resolved by Congress is whether products approved as biosimilars will be marketable and prescribed using unique or (bio)generic-like names. Innovator companies favor unique names, with this facilitating patient safety and post-market surveillance (and also maintaining their products’ branded identity). Biosimilar interests favor more generic-type names, such as names rather similar or even the same as the reference product, with this facilitating use of these products rather than the reference product and the lower costs that biosimilars are expected to provide (and allowing biosimilars to avoid much expensive brand-specific marketing, in some cases even being automatically substituted for the reference product). |