Pfizer: Employing PAT for Bulk Drying
Batch manufacturing is inherently difficult due to the stop, rebuild, and restart nature of the processes involved. Toss in real, potentially lethal, risks due to process inconsistencies, and its easy to see why the pharma manufacturing space is riddled with expensive and time-consuming QA/QC testing. With little or no on-line instrumentation to guide them, pharmaceutical manufacturing unit operators rely on SOP recipes to tell them how to process the step and the QA/QC lab to tell them if theyve done it right.
The FDAs Process Analytical Technology (PAT) initiative is an enabling directive to move the manufacturing end of the pharmaceutical business into a more on-line analytics model as typified by todays modern hydrocarbon processing or semiconductor manufacturing facility. The availability of near real-time measurements allows the operator to streamline the process or correct processing errors as they occur. The logical result of the use of on-line analytics is to automate the process itself by using collected data from any number of sources to model a good day in order to be able to predict, or fix, a day that has gone, or is rapidly going, bad. In this case, while the QA/QC lab may be necessary to do spot-checking from batch to batch, it will be the information coming from the on-line instrumentation that will trigger the release of the product or initiate the next step in the process.
While the ultimate end game hasnt yet been reached in pharmaceutical manufacturing, there is no doubt that the PAT initiative has spurred the use of on-line analytics in the sector. Even the tentative use of these instruments will begin to reveal the inner workings of the various unit operations with previously unseen levels of detail.
Bulk Product Drying
One of the, if not the, most time consuming of any of the unit operations is the bulk drying of either intermediates or APIs. There are generally large amounts of solvent-laden product involved and these solvents must be removed to a certain level prior to moving the product to the next step in the operation.
There are any number of dryer configurations available to accomplish the task. Vacuum dryers working down to 10 mbar absolute are very common as are combination dryers that use both pressure and vacuum to facilitate the drying process. The dryers can be equipped with various mechanical enhancements (stirring paddles; centrifuging action) designed to assist the process. Inert gas purging may also be performed to provide a measure of explosive protection or to keep filters and pumping paths cleared of product.
Without the benefit of on-line analytical data, this important production step has historically been done by using a time-temperature-pressure recipe with periodic QA/QC testing to chart the progress of the process. Of course there are variations to this recipe depending on the site and the product, but the path to a dry product has traditionally been a fairly simple affair. Simple, perhaps, because of the perceived simplicity of the process itself.
But what if the simple approach leads to a failure in the process that isnt as simple as the product not being dry enough but a failure that leads to the questioning of the quality of the drying process itself?
A Case Study
Process mass spectrometry has proven to be a remarkably effective way to monitor and streamline the drying process. With multiple point sampling, construction geared towards the industrial environment, and very high sensitivity when working with common pharmaceutical solvents, process MS can cut drying times by 50% or more, and can provide a wealth of information abut the drying process itself.
While the majority of these instruments are being used in API production and R&D/Pilot facilities, we were recently approached by Pfizer, Inc., which needed to investigate a possible drying problem that was leading to a failure of the product (a coated pill) during dissolution testing. While the product was in fact dry (with acetone as the primary residual solvent) it was beginning to appear to the group that not only was dry an important parameter, but how it got that way was equally as important.
Fig 1. Front view of walk-in tray dryers
In this case, the dryer in question was a tray type with an internal volume of roughly 480 cubic feet and there were three such dryers in operation (Figure 1). Each dryer could accommodate two carts carrying the pills on perforated trays. The carts were a very tight fit in the dryer leaving little space on either side of the cart.
The dryers were operated at slightly above atmospheric pressure and were of the flow-through type. Air from an outside handler/conditioner entered (~1000 cfm) from the top of the dryer and was pushed through a perforated side wall. From this wall, the heated air flowed over and through the trays on the cart and exited through another perforated wall on the opposite side of the dryer.
While these dryers had been characterized with regard to temperature distribution, it was felt that there was an uneven drying taking place due to possible stratification of the airflow as it moved through the trays. An Ametek Promaxion process mass spectrometer was used to determine whether there was any stratification taking place and, furthermore, whether this stratification was involved in the finished product non-uniformities.
The Installation
Each dryer was equipped with eight sampling pointsone each in the dryer inlet and exhaust plenums, and six points in the dryer volume separated equidistantly from top to bottom and front to back in the dryer (Figure 2).The sample tubes were 1/8 SS and were routed behind the perforated panels and up and out through the plenums as part of a multiple tube heated bundle that was maintained at 100°C to guarantee solvent mobility (Figure 2). This sampling arrangement provided a spatially differentiated look at each dryer along with totalized dryer input and output solvent levels by using the inlet and outlet sample points.
Fig 2. Heated sample tube bundle exiting the dryer
The mass spectrometer was installed approximately 90 away from the dryer suite (Figure 3). The heated bundles were routed above the instrument in the space above the dropped ceiling.
The analysis was done by stepping the mass spectrometer through each sample point in each dryer in a programmed sequence. Dryers not in operation were removed from the sampling sequence as needed. The major constituent of interest, acetone, was monitored at mass 43. Argon at mass 40 was also included in the analysis to indicate proper mass spectrometer operation. As with most drying operations, absolute sampling speed from point to point is of little concern due to the long duration of the process itself. In this case, each sample point was accessed every 20 seconds for a total loop time of approximately 8 minutes. Moisture removal was also monitored by using mass 18.
Results and Conclusion
Fig. 3. Promaxion process mass spectrometer
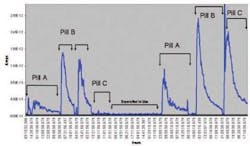
The fundamental concept behind the use of the mass spectrometer in this application is that the baseline of the mass spectrometer at the measurement mass for a given solvent will always be significantly lower than the ion current signal developed when the product is actually dry to spec. Figure 4 shows this situation clearly since the MS was continually monitoring the dryers even though no product was present. The baseline of the mass spectrometer is, in fact, several decades down from the ion current signal at the time the product was dry. Indeed, in each of the profiles presented in Figure 4, loss on drying testing confirmed that each batch had been dried to within the product specifications.
Fig 4. Overall Acetone drying curves from 6 batches
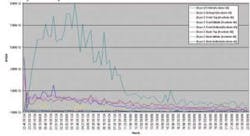
The initial supposition of the group concerning the stratification of airflow in the dryer is confirmed by the data in Figures 5 and 6. Figure 5 shows the ion currents developed for the acetone across all of the data points in one dryer for one batch of product. As expected, the mass spectrometer shows no significant amount of solvent in the incoming air stream, but clearly shows signal differences within the dryer from front to back and top to bottom. The dryer profiled in Figure 6 was fully loaded with product and solvent activity at every sample point can be seen with the differing solvent levels at each sample point indicating some sort of non-uniformity in the dryer air distribution.
Fig 5. Acetone response from individual sample points (Dryer Full)
Further confirmation of the laminar, or stratified, airflows in the dryer can be seen by examining Figure 6. In this case, the dryer was not fully loaded so that only a few of the sample points inside the dryer were actually close to a product tray. One would expect that at the high airflows found in these dryers and the volumes involved that there would be a good amount air mixing in the dryer itself. The data clearly shows this not to be the case. Even though all of the sample points were only several feet away from all of the areas where the trays would normally be, only those sample points that were right in line with the trays showed any solvent activity.
Fig 6. Acteone response from individual sample points (Partially filled dryer)
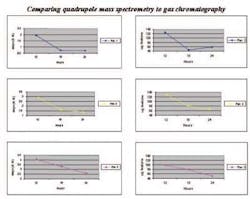
Finally, Figure 7 shows the correlation between the current validated loss on drying test and the results from the mass spectrometer headspace analysis. It can be seen from this graph that it would certainly be possible to pinpoint the exact time that the product has dried to specification by using only the mass spectrometer headspace analysis.
Whether any pharmaceutical manufacturing step can be called simple is a discussion for another day. One would have to admit, however, that the process of using vacuum, or heat, or agitation, or any combination thereof to dry a product, would look to be, on the surface, a fairly simple thing.
Fig 7. Correlation of GC and MS data
This work has shown that the application of on-line analysis to any of the pharmaceutical unit operations, regardless of the perceived complexity of the operation, will provide a wealth of information that can be used to potentially resolve manufacturing issues that may arise several steps away from where the measurements actually took place. This particular work is ongoing with other data being brought into the mix to fine-tune the manufacturing process. A complete solution will still take time to develop, but the on-line data can only help to speed that process by revealing conditions that would otherwise have been missed.