Traceability and genealogy are critical to manufacturers in all industries, but in regulated environments, they are just a prerequisite for staying in business.
Aberdeen’s recent cross-industry benchmarking survey (Traceability and Genealogy in Manufacturing) examined practices in various industries to determine which manufacturers had the best practices and could be considered Best in Class (BIC). The survey found that pharmaceutical manufacturers are more likely than Best-in-Class (BIC) manufacturers to adopt manufacturing execution systems (MES), but may still have some work to do before achieving BIC performance.
This brief report will examine the additional capabilities that drug manufacturers need to adopt to fill this performance gap. In November 2007, Aberdeen Group surveyed manufacturing executives at 54 pharmaceutical companies to understand their traceability and genealogy capabilities. To better understand the practices adopted by these manufacturers, Aberdeen analyzed the pressures driving them to focus on traceability and genealogy, the strategic actions they are taking in response, the business capabilities they possess, and the technologies that they are using to support these efforts.
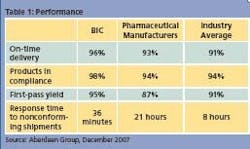
Pharmaceutical manufacturers are twice as likely to be driven by compliance pressures compared to Best-in- Class manufacturers. However, some of them may ignore important secondary pressures, such as the need to reduce the cost of poor quality and to mitigate the risk of product recall. Survey results suggest that some drug manufacturers may be so focused on complying with regulatory standards that they are not paying attention to these other factors. For example, these manufacturers are falling behind the industry average when it comes to first-pass yield (87% vs. 91%) and well behind the 95% performance of BIC manufacturers.
The table’s results are telling. Pharmaceutical manufacturers are operating at the Industry Average level and are approaching BIC performance when it comes to products manufactured while in compliance. These performance results are well aligned with their priority on compliance with regulatory standards. On the down side, it takes drug manufacturers 21 hours, on average, to respond to nonconforming shipments. The industry average is 8 hours, but for BIC manufacturers, the time required is just a little over a half an hour. An average response time of 21 hours tells us that pharmaceutical manufacturers are more focused on internal processes, and suggests that they still need to gain visibility within their plants and throughout the supply chain to have realtime information about the status and quality of their products until they reach the final consumer.
Automating Traceability and Genealogy
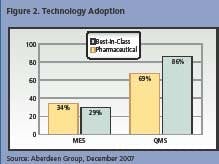
Aberdeen’s survey found a direct correlation between technology adoption and BIC performance. Automating traceability and genealogy is fundamental for manufacturers to eliminate the error-prone manual process of working with spreadsheets. Aberdeen researchers have determined that the combination of a manufacturing execution system (MES) and a quality management solution (QMS) offers comprehensive process and product traceability information that is critical for BIC manufacturers to perform at an elevated level. While drug manufacturers are ahead of their BIC counterparts in their use of MES, the adoption rate is still extremely slow. In addition, drug makers are 25% less likely than BIC manufacturers to adopt a QMS.
When investing in technology, pharmaceutical manufacturers should look at specific technological capabilities that differentiate BIC performance. Nonconformance/Corrective and Preventive Action (NC/CAPA), Statistical Process Control (SPC), process traceability and automated workflow modules are key to establishing a standardized process for traceability as well as handling nonconformance across the enterprise. Also important are supplier scorecards and quality dashboards, both to automate data collection from the plant floor and from suppliers, and to present that information to the right person at the right time in the right format.
This capability is a huge contributor to BIC manufacturers’ ability to respond to a nonconformance incident within 36 minutes vs. 21 hours for pharmaceutical manufacturers. Yet, drug makers are less likely than BIC manufacturers (see Figure 3, below) to adopt all of the aforementioned modules, which are vital to an efficient traceability process. Adopting these modules allows manufacturers to capture critical information on the product and to track and trace it as the product moves down the manufacturing line.
Standardization on the use of these modules across the enterprise will enable key personnel throughout the organization to get real-time visibility into the manufacturing process as well as the supply chain.
Required Actions
Our research suggests that the following steps will help more drug manufacturers improve operational performance and ensure future success for their traceability and genealogy initiatives.
- Automate collection of quality data from the plant floor and suppliers and use it as actionable intelligence Adopt technology to automate collection of quality data from the plant floor and from the suppliers. Automating the data collection processes across the entire production process, including the supplier base, will provide key capabilities to analyze and escalate quality and nonconformance issues with actionable intelligence and help improve responsiveness.
- Adopt MES and QMS solutions that provide core track and trace capabilities Aberdeen’s analysis has found that manufacturers adopting MES and QMS are more likely to perform at a BIC level. Pharmaceutical manufacturers that still have not adopted these solutions should do so, with a focus on the specific modules mentioned above.
- Establish real-time interoperability between plantfloor automation, MES, QMS, ERP and SCM. Traceability is about providing visibility into the entire lifecycle of a product throughout its various operational stages. The final step for pharmaceutical manufacturers is to create real-time interoperability among plant floor automation, MES, QMS, and enterprise applications (ERP, SCM among others). Investing in interoperability across technology will enable pharmaceutical manufacturers to gain real-time visibility into product and process data across the value chain, resulting into improved operational performance.
About the Author
Mehul Shah is a research analyst with The Aberdeen Group.