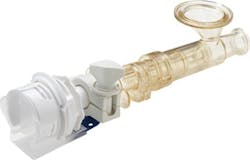
Colder Products: AseptiQuik STC provides flexibility for hybrid stainless and single use systems
Colder Products Company announces the release of the new AseptiQuik STC for use in biopharmaceutical processes using a hybrid combination of stainless equipment and single-use systems. The combined AseptiQuik sterile connector and Steam-Thru II SIP connector gives biopharmaceutical manufacturers increased flexibility in process design and plant configuration, lowering overall production costs and reducing time to market.
The union of the two connectors into a single unit via a sanitary clamp creates a combination product that allows an AseptiQuik sterile connection to be steamed(SIP) on to stainless equipment. After the SIP cycle, a wide range of single-use systems can be connected to the AseptiQuik STC, presenting manufacturers with even greater flexibility for hybrid stainless steel and single-use processing.
The AseptiQuik STC connector can be mounted directly to the stainless steel vessel via either a 3/4" or 1-1/2 " sanitary termination. The SIP process can be done in advance allowing a quick and easy sterile connection to the AseptiQuik half without having to wait 30-60 minutes for SIP prior to feed or harvest. The AseptiQuik STC connectors are offered in both a standard version for gamma sterilization and a high temperature (HT) version for autoclave sterilization.
Colder’s bioprocessing connections are manufactured in an ISO Class 7 certified cleanroom and meet USP Class VI and ADCF requirements. Contact Colder for validation test summaries, biocompatibility and extractables data.