- Rapid Expansion of Supercritical Solution (RESS),
- in which a drug is dissolved in an SCF such as CO
- and then sprayed into a collection chamber. The solvent is then rapidly removed, resulting in well-defined, uniform drug particles. RESS is a simple and effective technique, but is restricted by the limited solubility of drugs in SCFs.
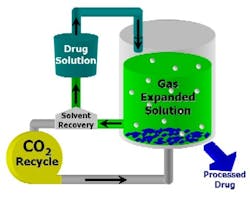
- Gas Anti-Solvent (GAS)
- is another popular technique, shown in
- (
- ). In this case, a gas such as CO
- is added to an organic solution of a desired drug, resulting in the expansion of the organic solvent and the precipitation of uniform drug particles. The precipitation is easily tuned by adjusting temperature and CO
- pressure, allowing close control of particle size and morphology. GAS takes advantage of the organic solvent’s strength coupled with the tunable solvent expandability. However, it requires semi-batch operation.
- Supercritical Anti-Solvent (SAS) and Solution Enhanced Dispersion by Supercritical fluids (SEDS)
- allow for continuous operation and control. In both of these processes, the drug solution is sprayed into the SCF or mixed with the SCF and sprayed into a collection vessel. Nozzle design and inlet stream flow rates can be adjusted to control the process while utilizing the solvent’s tunability.
- Gas eXpanded Liquids (GXLs),
- organic solvents mixed with carbon dioxide gas, which expands their volume by a factor of 10 or more. Nutraceuticals production has moved in the direction of GXLs, by using ethanol cosolvent as a polar modifier with scCO
- . This results in a solvent medium that can combine synthesis and product recovery in a simplified and efficient “one pot” system.
- Near-critical Water (NCW),
- which offers organic solubilities and the potential for reversible acid/base catalysis.
- Organic/Aqueous Tunable Solvents (OATS),
- or water-soluble catalysts (including both aqueous organometallic complexes and enzymatic biocatalysts) that can perform difficult transformations on highly hydrophobic substrates.
- Miscible water/organic systems,
- where a catalyst is modified for aqueous solubility;
- Miscible poly(ethylene glycol)/organic systems,
- where a catalyst is modified for PEG solubility;
- Immiscible fluorous/organic liquid/liquid or solid/liquid systems,
- where the catalyst is modified for fluorous solubility;
- Immiscible water/organic systems
- involving phase transfer catalysts.
http://www.che.gatech.edu/ssc/eckert/.Dr. Christopher L. Kitchens is a Post Doctoral Researcher in the Eckert-Liotta Joint Research Group at Georgia Institute of Technology. He received a B.S. in Chemistry from Appalachian State University and a Ph.D. in Chemical Engineering from Auburn University, where he worked on nanoparticle synthesis and processing in tunable fluids.
Dr. Jason P. Hallett is a Research Engineer in the Eckert-Liotta Joint Research Group at Georgia Institute of Technology. He received a B.S. in Chemical Engineering from the University of Maine and a Ph.D. in Chemical Engineering from Georgia Institute of Technology, where he worked on novel methods for homogeneous catalyst recycle.References
Dr. Jason P. Hallett is a Research Engineer in the Eckert-Liotta Joint Research Group at Georgia Institute of Technology. He received a B.S. in Chemical Engineering from the University of Maine and a Ph.D. in Chemical Engineering from Georgia Institute of Technology, where he worked on novel methods for homogeneous catalyst recycle.References
- McHugh, M; Krukonis, V., Supercritical Fluid Extraction: Principals and Practice, Butterworth Publishers, Stoneham, Mass., 1986.
- York, P.; Kompella, U.; Shekunov, B., Supercritical Fluid Technology for Drug Product Development. Drugs and the pharmaceutical sciences ; v. 138 M. Dekker, New York, 2004.
- Staks, C.; Liotta, C.; Halpern, M., Phase-Transfer Catalysis: Fundamentals, Applications, and Industrial Perspectives. Chapman & Hall, New York, 1994.
- Xie, X.; Brown, J.; Joseph, P.; Liotta, C.; Eckert, C.,“Phase-Transfer Catalyst Separation by CO2 Enhanced Aqueous Extraction”, Chemical Communications, 2002, 1156.
- Eckert, Charles A.; Liotta, Charles L.; Bush, David; Brown, James S.; Hallett, Jason P. “Sustainable Reactions in Tunable Solvents.” J. Phys. Chem. B 2004, 108, 18108.
- Brown, J.; Gläser, R.; Liotta, C.; Eckert, C.,“Acylation of Activated Aromatics without Added Acid Catalyst”, Chem. Commun., 2000, 1295-1296.
- Chandler, K.; Deng, F.; Dillow, A.; Liotta, C.; Eckert, C., “Alkylation Reactions in Near-Subcritical Water in the Absence of Acid Catalysts,” Ind. Eng. Chem. Res., 1997, 36, 5175.
- Nolen, S.; Liotta, C.; Eckert, C.,“The Catalytic Opportunities of Near-Critical Water: A Benign Medium for Conventionally Acid and Base Catalyzed Organic Synthesis,” Green Chem., 2003, 663.
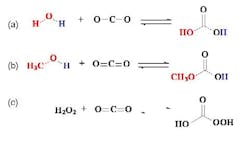
- West, K.; Wheeler, C.; McCarney, J.; Griffith, K.; Bush, D.; Liotta, C.; Eckert, C.; “In Situ Formation of Alkylcarbonic Acid with CO2” J. Phys. Chem. A, 2001. 105, 3947.
- Xie, X.; Liotta, C.; Eckert, C., CO2-Catalyzed Acetal Formation in CO2-Expanded Methanol and Ethylene Glycol. Ind. Eng. Chem. Res. 2004, 43, 2605.
- Chamblee, T.S., R.R. Weikel, S.A. Nolen, C.L. Liotta, and C.A. Eckert, “Reversible in situ acid formation from beta-pinene hydrolysis using CO2 expanded liquid and hot water” Green Chem., 2004, 6.
- Nolen, S.; Lu, J.; Brown, J.; Pollet, P.; Eason, B.; Griffith, K.; Glaser, R.; Bush, D.; Lamb, D.; Eckert, C.; Liotta, C.; Thiele, G.; Bartels, K.,“Olefin Epoxidations Using Supercritical Carbon Dioxide and Hydrogen Peroxide Without Added Metallic Catalysts or Peroxy Acids,” Ind. Eng. Chem. Res., 2002. 41, 316.
- West, K.; Hallett, J.; Jones, R.; Bush, D.; Liotta, C.; Eckert, C. "CO2-Induced Miscibility of Fluorous and Organic Solvents at Ambient Temperatures." Ind. Eng. Chem. Res., 2004. 43, 4827.
- Lu, J.; Lazzaroni, M.; Hallett, J.; Bommarius, A.; Liotta, C.; Eckert, C. “Tunable Solutions for Homogeneous Catalyst Recycle.” Ind. Eng. Chem. Res. 2004, 43, 1586.
About the Author
Charles A. Eckert
Charles L. Liotta
Sign up for our eNewsletters
Get the latest news and updates