Intranasal delivery is an exciting target for drug delivery as it allows for both systemic and topical drug absorption due to its large surface area, extensive blood vessels and the possibility of direct access to the central nervous system (CNS).
However, any promising treatments can only advance as fast as the models that are used to evaluate them. Here, the latest advancement in the modeling of intranasal delivery — the reconstituted nasal epithelium (RNE) model — is summarized along with the long-standing traditional techniques that have helped pharma companies de-risk and expedite the delivery of intranasal drugs.
An intro to the nose
Delivery into the nose is relatively painless, the onset of drug action can be rapid, and formulations can be administered in emergency situations, such as with Narcan intranasal spray for opioid overdose.
A major contributor to the potential of the nose as a drug delivery route is the structure of the epithelial surfaces, which can be divided into several general regions. The largest of these is the nasal cavity, which has a convoluted surface to slow incoming air and promote particulate deposition on the nasal mucosa. It consists of three rigid shell-like protrusions known as turbinates. These bony structures have a high density of blood vessels to provide warmth and moisture, which serves to condition the air for optimal gas exchange. As such, the nasal cavity makes an excellent target for drug delivery because droplets from sprays or particulates from powders, which encounter the turbinates, adhere to the mucosal surface and thereby gain access to the vasculature.
One limitation is the protective layer of mucus on the epithelium and ciliated epithelial cells, which can rapidly capture and sweep drugs to the oropharynx where they are cleared by swallowing. To avoid this, formulations need to be designed to allow for optimum drug release and absorption.
Another target for drug delivery within the nose is the olfactory mucosa, a specialized region situated above the nasal cavity. The olfactory neurons and supporting sustentacular cells in this region provide direct access to the CNS, bypassing the blood-brain barrier and making it possible to deliver a drug within seconds. The mechanism by which this happens is still a subject of differing opinion but is likely to involve drug transport through the paracellular space between olfactory axons, or via the conduit-like lamina propria in which they are bundled.
Traditional intranasal screening models
Numerous models have been developed for testing intranasal formulations. Among the earliest of these was the differentiation of airway epithelium in air-liquid interface (ALI) cultures, which first appeared in literature in the early 1980s. However, such models have historically been highly complex and difficult to reproduce, and reliable sources of primary cells are hard to obtain.
Another traditionally successful screening technique involves excised nasal animal mucosa (e.g. sheep) mounted in a static diffusion cell. In this model, formulations are applied to the apical side of the tissue, and receptor solution is sampled from the basolateral side to assess the performance of the formulation.
Such models have assisted in the development of nasal drug formulations for decades. However, although the passive barriers such as basement membrane and matrix binding remain intact, the active components such as tight junctions, mucus production, cilia and other cellular activities do not, and thus, these models do not provide a true representation of nasal drug absorption.
Improvements to traditional models
To build on these models, a reconstituted nasal epithelial model has been developed.
In this model, nasal epithelial cells are harvested from a donor using a nasal swab and suspended in a gentle proteolytic media bath. The epithelial cells are then isolated from resident immune cells and fibroblasts. Subsequently, they are >seeded onto porous membranes and expanded in monolayer culture using a proprietary epithelial growth media. Over about a week, the cells expand and become fully confluent, beginning the differentiation process. At this point, the proprietary media is changed, and the apical surface is kept dry to promote full differentiation, which occurs over three or four further weeks. Over this time, the epithelial cells adopt a pseudostratified columnar compound epithelial layer. These cells exhibit mucus production, ciliary activity and tight junctions, and this architecture typically persists for approximately four weeks.
An important aspect in determining the success of this model is the development of tight junctions present in the nasal cavity, which form an electrochemical barrier between the basolateral and apical chambers. Thus, the existence of these tight junctions can be determined from trans-epithelial electrical resistance (TEER), a measurement of resistance across a cellular layer. TEER can be measured immediately before administering a formulation to the RNE model, to ensure that only intact and well-differentiated constructs are used in analysis.
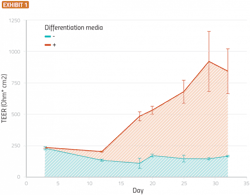
Current studies suggest that tight junctions can develop to a stable state over about three weeks while differentiation media is present (Exhibit 1).
Primary human nasal epithelial cells were seeded onto a permeable membrane suspended in media. The cells were allowed to grow until confluent (approximately 10 days), at which point they were switched to an air-liquid interface and provided with differentiation or growth media. The figure shows transepithelial/transendothelial electrical resistance plotted vs time for cells treated with growth media (light blue) or differentiation media (red). Error bars are standard errors of the mean (SEM), n=5.
Testing an RNE model
Several clinical studies have compared intranasal to IV administration of oxycodone and buprenorphine. To determine if the same qualitative trends translate from such studies to the RNE model, researchers pre- pared buprenorphine and oxycodone formulations that closely mimicked the formulations used in these clinical studies.
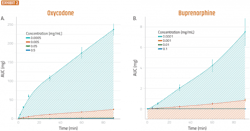
Clinically, the oxycodone formulation was reported to have a several-fold higher maximum active pharmaceutical ingredient (API) concentration (Cmax) than the buprenorphine formulation. It was also found that the RNE model produced similar results to the clinical studies, with only minor differences in the magnitude of differentiation (Exhibit 2).
To further determine formulation discrimination in the RNE model, dose-response for the oxycodone formulation was evaluated. Four concentrations of oxycodone formulation were prepared and administered apically to RNE constructs (n=5). At designated intervals, basolateral media was sampled and replenished to constant volume. At each time point, the total cumulative amount of API was calculated. The total cumulative amount at all time points past two minutes showed a linear response to formulation concentration (R2 > 0.90) and thus a dose-response for the oxycodone formulations was observed.
Taking the RNE model forward
It is always desirable to develop the next, more useful model to advance drug discovery and formulation development. The development of an RNE model has increased the range and potential of available models for testing intranasal formulations. In addition to more closely resembling the nasal mucosal barrier than traditional animal tissue models, RNE cultures allow for additional analyses that are not possible using ex vivo tissue.
In addition, the RNE model can be adapted for bronchial epithelium, allowing for the testing of a host of new formulation types, such as those for the treatment of asthma, COPD, bronchiectasis and cystic fibrosis. In the case of cystic fibrosis (and other genetic diseases), epithelial constructs can be grown from cells collected from the affected patient population during routine medical care.
Another application of RNE models is co-culturing RNE and other airway epithelium. One example is co-culturing RNE with inflammatory cells in invasion assays to compare the performance of anti-inflammatory and antibiotic drugs. Reconstructed airway tissue can also be co-cultured with airway smooth muscle to compare the efficacy of drugs treating airway hyperresponsiveness in asthma. Due to the long-term viability of such RNE constructs, they can also be used to model the effects of irritants, such as smoke, diesel exhaust particles and other environmental particulates, and the recovery of the airways from injury.
10 μL of Oxycodone (A) or Buprenorphine (B) in saline solution was applied to the apical side of well-differentiated RNE cells. At the indicated times, the basolateral media was collected and replaced with an equal volume of fresh, pre-warmed media. Total permeated quantity at each timepoint (AUC) was calculated and plotted vs time. Error bars are standard errors of the mean (SEM), n=5.
Finally, RNE models can be used to monitor nasal infection. The COVID-19 pandemic has highlighted the importance of good models of infection and pathology, and the role of the nasal epithelium in infection and viral propagation. Models of coronavirus infection in the nasal epithelium using reconstituted nasal epithelium have also been developed.
Modeling infection in RNE bypasses many well-known shortcomings of cell lines (such as HBE4 and A549), as well-differentiated primary cells more closely mimic in vitro physiology, cell signaling and architecture. Although RNE models don’t eliminate artificial selection due to conditions in the host cell (such as protease and receptor expression), they reduce this effect.