Quantifying technology’s impact on vaccination rates
Every year, businesses and organizations spend billions of dollars on vaccine development, with millions going specifically toward the development of new vaccine technologies. These technologies can offer simpler and safer administration methods, improved thermostability, and fewer required doses, which can in turn offer wide-ranging benefits such as lower costs for vaccine storage, reduced chances for adverse events, and increased efficacy.
Many of these benefits are captured during the development process through laboratory experiments, clinical trials and health economic studies. One benefit that is rarely quantified, however, is the expected increase in vaccination coverage rates.
The design and development of vaccines is already incredibly expensive and complex — there are high capital costs associated with every decision along the development pathway. And the vaccine business relies heavily on scales to make it work. Having a strong, data-backed understanding of how many vaccines can be sold in any given market before starting to manufacture a vaccine is incredibly important.
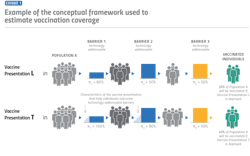
For decision-makers in the vaccine development and manufacturing space, it’s important to understand that vaccine technologies play an especially significant role in determining demand and coverage rates. For example, say a country has access to a microarray, patch-based vaccine delivery system that could replace the standard syringe and needle delivery system. What might that do to vaccination coverage rates in that country?
Initially, the answer might be “not much.” But once you consider the operational implications of who is administering a particular vaccine, the implications for the delivery system can change dramatically. For instance, a vaccine that requires a mid-level skilled health care worker such as a nurse to administer compared to a less skilled worker, such as a community health worker, could lead to significantly higher coverage rates, especially in countries with fewer mid-level skilled health care workers.
Anticipating demand for different vaccine presentations early in the development process is an incredibly powerful tool that can be utilized for more intentional decision-making. The type and magnitude of potential benefits make it possible to channel resources toward vaccine technologies and presentations with greater promise.
Key barriers to vaccination
The COVID-19 pandemic shined a huge spotlight on the barriers to vaccination that exist for many people across the world. For example, the refrigeration requirements for some of the COVID-19 vaccines were a significant obstacle in many countries. Many ministries of health simply couldn’t afford the ultra-cold storage freezers required for the vaccines, or the electrical grids were not reliable and stable enough to keep the freezers continuously running. Similarly, the need for multiple doses and limited access to skilled health workers created additional challenges toward full inoculation.
The result? Staggering levels of COVID-19 vaccine inequity across the globe. Reducing such barriers when developing vaccines will not only help to increase coverage rates and demand, but it is also critical in protecting lives.
Barriers to vaccination come in two main categories: those directly addressable by vaccine technologies (‘technology-addressable’) and barriers that are ‘non-technology-addressable’. The non-technology-addressable barriers are factors inherent within a country’s current health system. For instance, this could include factors like the number of physicians and/or nurses, conflicts such as civil war and the amount of government health care funding available, to name a few. With non-technology-addressable barriers, there is an assumption that the environment will not change drastically over time between the current state and a future state. With those inherent non-technology-barriers factored in, it’s then possible to estimate the potential impact of reducing the technology-addressable barriers.
As exemplified earlier with the patch-based vaccine, some technologies could mitigate or remove barriers, leading to increased demand for vaccines and higher coverage rates. Global health partners in this space, including the World Health Organization (WHO), defined five technology-addressable barriers:
1. Vaccine schedule: The probability that a member of the vaccine-eligible population does not receive vaccination due to an inability to comply with the existing vaccine schedule.
2. Temperature storage requirements: The probability that a member of the vaccine- eligible population does not have access to vaccines properly stored in a functional cold chain environment since their manufacture.
3. Administration requirements: The probability that a member of the vaccine-eligible population does not have access to an individual who can administer a vaccine using the most complex administration method.
4. Acceptability of presentation: The probability that a member of the vaccine-eligible population (or caregiver) exhibits vaccine non-compliance due to specific characteristics of the vaccine presentation. (For example, potential issues due to the use of pork products in the manufacturing process of the vaccine.)
5. Doses per container: The probability that a member of the vaccine-eligible population is refused vaccination due to provider’s unwillingness to open a container.
Our research identified a sixth technology-addressable barrier: Number of doses. This was necessary to include since data shows that the number of people fully vaccinated drops off as the number of required vaccine doses increases. The assumption here is that a vaccine with more than one dose requires an individual to overcome the other five barriers multiple times, further reducing coverage rates.
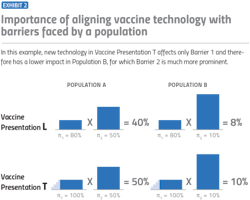
Each of these technology-addressable barriers includes a range of scenarios and presentations that affect coverage rates, from low to high. In breaking down the effects into five different categories, including low, medium-low, medium, medium-high and high, we were able to build an effective model for estimating vaccine coverage rates. The model focuses especially on low- and middle-income countries (LMICs), but can be applied to any region of the world.
To further put this into perspective, let’s look at one of the biggest barriers of vaccination in African countries: temperature storage requirements. While we previously mentioned this as a barrier for the COVID-19 vaccine in particular, cold storage requirements for vaccines present a broader challenge for other vaccines as well. Requiring an unbroken frozen chain of -15 degrees Celsius or lower would create the highest barrier for vaccine coverage. However, making a vaccine that requires controlled temperature, with the ability to tolerate up to 40 degrees Celsius for at least three days, would have a large effect of up to 50% on coverage rates.
A model for estimating vaccine coverage rates
Context is everything when it comes to the impact of vaccine barriers on a population’s coverage rates. As noted, in certain countries, developing a vaccine that doesn’t require refrigeration could have a huge effect on the volume of vaccines one is able to sell. In other countries, where refrigeration is consistently available, that might not be the case.
As a vaccine manufacturer, there might be some intuitive guesses early on for what could increase demand. Wouldn’t it be helpful if we could make a vaccine that can be delivered by a community health worker instead of a nurse? Wouldn’t it be nice if we made a vaccine that was packaged with fewer doses per box? These are fine approximations at first, but they obscure the interaction between the characteristics of the vaccine with the characteristics of the environment. What is a country’s infrastructure? What does the country’s health care system look like, and how much would it benefit from different technological improvements?
The model turns a relatively simple assumption into numbers that show the increase in coverage rates. It produces something to the effect of, “If country X had access to vaccine presentation Y, it could achieve a vaccination coverage of Z%.” The model employs ‘probability theory,’ which essentially says if the probability of A happening is a, and the probability of B happening is b, the probability of both A and B happening is a x b. The math is slightly more complicated, but the general idea is that the probability of a person in country X with access to vaccine presentation Y getting vaccinated is the product of the probabilities of a person overcoming each of the six barriers defined above.
One benefit of this approach is that it can be used at any point along the vaccine development pathway. Whether someone is thinking about research and development or considering manufacturing vaccines for a particular region, this approach allows them to estimate the maximum demand for a specific vaccine presentation in different deployment contexts.
Take, for example, a vaccine for malaria. If you are trying to figure out the potential volume — measured by the potential increase in coverage rates — across the globe, this model allows you to input the vaccine presentation and see where the highest potential volume for that vaccine exists. This would help in determining where manufacturing can focus.
In a different scenario, there could be three presentations of a malaria vaccine — syringe, patch and aerosolized — and a commitment to manufacturing in, say, East Africa. The model can show which of the three presentations would have the largest potential volume, giving concrete numbers into how these vaccine technologies would affect coverage rates in the context of countries in that region. In both of these scenarios, this high-level view helps in directing focus and investment early in the decision-making process.
As mentioned, this model is particularly specialized in estimating vaccination coverage rates in LMICs for three reasons:
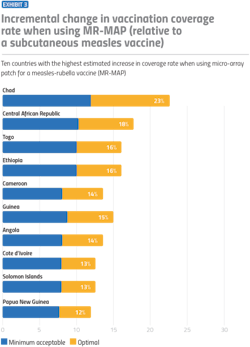
First, while access to vaccines for childhood illnesses, such as measles and polio has increased dramatically during the past 50 years, in many LMICs, vaccination rates are still well below targets. These lower rates signify an opportunity for vaccine manufacturers to reach these markets and increase coverage with new vaccines.
Second, LMICs are the types of data-limited environments in which an answer that relies on data is especially helpful, without having to invest significant funds towards deep market analysis.
Finally, a greater proportion of the barriers to vaccination in LMICs are technology addressable compared with high-income countries.
This approach of tackling technology-addressable barriers can also be expanded for use in any pharmaceutical or medical technology. In principle, one could extend this method to almost any product or service, whether it is health-related or not. What would need to change are the barriers and the definitions behind them. For example, when thinking about methods of getting an ultrasound, access to refrigeration is probably not a barrier to treatment, but access to reliable power through a stable electrical grid probably is.
The model in action
This model is currently in use with major donor organizations working in vaccine-related spaces, helping guide their investments. For vaccine manufacturers, it’s equally, if not more important, to consider how reducing these six technology-addressable vaccine barriers will affect coverage rates. Money and time are limited. Having an idea of a particular vaccine’s market share before taking the leap into an investment is strategically helpful.
While reducing technology-addressable barriers certainly helps increase coverage rates in both high-income countries and LMICs, there is a significant market and need for effective vaccines in the latter. Those with resources in the vaccine space should seriously consider developing more platforms that help reduce barriers for countries with the fewest resources.
The COVID-19 pandemic has made it clear that identifying ways to increase vaccine coverage rates across the world is critical to our survival as a global population.
For more information on the methodology discussed in this article, Pascale Leroueil can be reached at: [email protected].