The increasing frequency of prescription drug shortages in the United States can often be attributed to gaps in efficiency within the pharmaceutical supply chain. Multiple factors including market dynamics, loss of patent exclusivity, quality issues, disruption of raw material supply, inventory practices, natural disasters, and manufacturing problems can lead to an unstable drug supply.
Drug shortages can impact both the quality and economics of health care. Undesirable outcomes driven by the need to use alternatives during drug shortages have been observed with numerous classes of drugs, including vasopressors, anesthetics, anti-infectives and anti-neoplastics.
On the pricing side, an analysis of U.S. Food and Drug Administration pricing data from December 2015 to 2016 found that the price of generic drugs in limited supply that were produced by three or fewer manufacturers increased by an average of 20 percent. The price of generic drugs that were not in shortage over the same period increased by 9 percent.1
A number of solutions have been proposed to help alleviate drug shortages, including:
• Financial incentives to manufacturers that establish and maintain additional manufacturing capacity to prevent drug shortages
• Financial incentives to invest resources in manufacturing generic drugs with less desirable economics
• Mandated inventory levels for critical drugs
• New business models for stabilizing the supply of drugs that are subject to frequent shortages
Glass as a strategy
Recent innovations in glass packaging provide another strategy to secure the pharmaceutical supply chain by improving quality and increasing manufacturing efficiency. Improved glass packaging is particularly relevant to the issue of drug shortages — statistics show that injectables are the most frequently impacted therapeutics affected by shortages in the U.S. Between 2001 and 2018, injectables represented ≥50 percent of new shortages in 14 years of this 18-year time period.2
Glass innovations address issues that have historically limited the performance of glass packaging such as delamination, particulate generation and breakage.Glass delamination
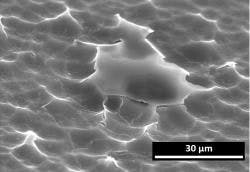
Delamination is a response primarily observed in converted tubing glass vials in which thin flakes release from the interior surface of the vial into the liquid formulation (see Exhibit 1). The propensity for delamination is dependent on multiple factors, although its origin is the surface chemical heterogeneity that is created during conversion of glass tubing to vials. Potential risks of delamination flakes include inflammatory responses that injure tissue, stimulation of undesirable immune responses, and/or tissue injury through occlusion of vasculature.3 As a result of these potential risks, drug lots affected by glass delamination are recalled, thereby impacting supply.
Subsequent work on new glass formulations has led to solutions that address the root cause of delamination.4 In particular, boron-free aluminosilicate glasses eliminate the volatilization-prone component leading to chemical heterogeneity and subsequent delamination. These aluminosilicate glasses also meet the hydrolytic performance requirements used for parenteral packaging as outlined by the United States Pharmacopeia (USP).
Glass particulate contamination
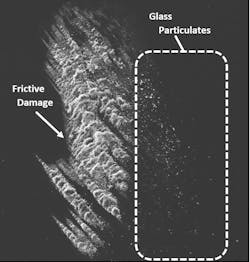
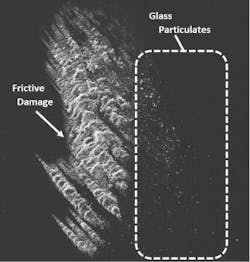
The presence of glass particulates is another frequent source of recalls.5 Frictive contact between vials and vial impact events during filling can generate particulates (see Exhibit 2). Complete breakage of a vial can also generate glass particulates. In either case, the resultant particles can lead to contamination, loss of product, and expensive recalls that jeopardize the supply chain. Potential health risks of glass particulate contamination of a parenteral product are similar to those related to delamination events.3
A second approach is to increase the vial’s inherent ability to withstand damage that generates particulates. Vials enhanced with a low coefficient of friction (COF) coating on the exterior surface can significantly decrease glass particulate generation on a filling line by reducing the forces generated by vial-to-vial contact. In one study, low COF vials were found to provide a 96 percent reduction in peak levels of particles greater than 0.5 µm in size.6
Breakage during fill-finish manufacturing
Fill-finish operations are generally the most capital-intensive stage of the drug manufacturing process — heavy asset utilization is therefore critical to profitability. Product that has reached the fill-finish stage of manufacturing has the greatest value. Process changes that have the potential to negatively impact yield are generally avoided to mitigate risk. As a result, adoption of new technologies within fill-finish operations has historically been conservative and incremental.7 These manufacturing innovations can also be limited by the glass packaging used on the filling line.
Glass packaging performance in fill-finish operations is evaluated by multiple metrics. Glass particulate contamination is one type of defect that occurs during filling operations. If particulates are detected during post-filling inspection, the resulting root cause investigations can lead to significant downtime. Other glass-related factors that impact yield include tip overs and broken vials that require operator intervention and/or line stoppage in addition to loss of product.
Low COF coatings provide additional benefits that improve manufacturing throughput. The ability of coated vials to smoothly flow on a line reduces tip overs and jams. Coatings also provide protection against the creation of strength-limiting surface flaws that render a vial more susceptible to breakage. The benefits of damage resistance extend to the inspection stage — less damage during filling leads to fewer cosmetic defect rejects.
Additional features can be designed into a glass vial to augment breakage resistance. One solution is to chemically strengthen the glass surface using an ion exchange process. The resulting compressive stress layer imparts additional breakage resistance to a vial by closing surface flaws and resisting applied tensile stresses encountered on a filling line.
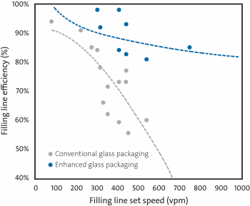
The cumulative benefits of using new vial technology with low COF coatings and chemical strengthening have shown an average throughput improvement of >20 percent for filling lines operating at the same set speed used for conventional vials. As a result, pharmaceutical companies can realize an immediate boost in capacity without investing in expensive fixed assets or increasing their manufacturing footprint. Contract manufacturing organizations that are frequently engaged to fill the additional demand of pharmaceutical companies can also benefit from the increased efficiency and improved yield of quality product that is enabled by innovative glass packaging.Capacity can also be hypothetically increased by operating filling lines at faster speeds. The efficiency of current high-speed filling lines is typically 60-70 percent with extensive breakage throughout the process, and the efficiency continues to decline significantly when using conventional glass packaging. The improved machinability and breakage resistance of enhanced glass packaging enables a new, previously unattainable trajectory in efficiency and throughput relative to conventional packaging, as shown in Exhibit 3.
A win-win
The drug shortage crisis in the U.S. can impact the quality and economics of health care. Numerous solutions to reduce drug shortages have been proposed, many of which focus on public policy initiatives. An alternative approach to stabilizing the supply chain enhances manufacturing with technologies that improve drug quality, increase throughput and reduce waste. Innovative glass vials engineered with new features such as low COF coatings and chemical strengthening are a promising technology and a potential win-win for patient safety and manufacturers looking to improve quality and increase efficiency while maximizing the utilization of capital-intensive manufacturing equipment.
References
1. Hernandez, C.B. Good, A.S. Hesselheim, W. Shrank (2018). Changes in drug pricing after drug shortages in the U.S.. Annals of Internal Medicine, Article accepted for publication, doi: 10.7326/M18-1137.
2. Drug shortages statistics. ASHP. Accessed on August 26, 2019.
3. S. Bukofzer, J. Ayres, A. Chavez, M. Devera, J. Miller, D. Ross, J. Shabushnig, S. Vargo, H. Watson, and R. Watson (2015). Industry perspective on the medical risk of visible particles in injectable drug products. PDA Journal of Pharmaceutical Science and Technology, 69: 123-139.
4. R.A. Schaut, J.S. Peanasky, S.E. DeMartino, and S.L. Schiefelbein (2014). A new glass option for parenteral packaging. PDA Journal of Pharmaceutical Science and Technology, 68: 527-534.
5. S. Tawde (2014). Particulate matter in injectables: Main cause for recalls. Journal of Pharmacovigilance, 3: 1-2.
6. C. Timmons, C.Y. Liu, and S. Merkle (2017). Particulate generation mechanisms during bulk filling and mitigation via new glass vial. PDA.
7. R.A. Rader, E.S. Langer. Fill-finish innovation, Contract Pharma, Accessed on August 28, 2019