Small molecule kinase inhibitors are a class of drugs used in the treatment of cancer and autoimmune diseases. The U.S. Food & Drug Administration has approved more than 45 kinase inhibitors since they first reached the market in 2001 with Gleevec. The total revenue generated by kinase inhibitor drug products worldwide now exceeds $30 billion per year.1 With seven approvals in 2017 and eight in 2018, and more than 250 currently in development, it appears the number of kinase inhibitor therapies will only increase in the coming years.
Despite their abundance in cancer therapy, kinase inhibitor drugs are often plagued by challenging physicochemical and biopharmaceutical properties, including high dose, low water solubility and high lipophilicity, which can lead to unoptimized absorption and pharmacokinetic properties. When uncorrected by an enabling formulation approach, poor drug absorption can result in a high pill burden, food-label restrictions and absorption-related drug-drug interactions in patients. The net effects of these dosing and pharmacokinetic issues are reduced patient compliance and inferior therapeutic outcomes.2,3 Yet only three of the 45 FDA-approved kinase inhibitor products utilize an enabling formulation approach, in part because the treatments are often fast-tracked to the market, which can limit scope to explore alternative formulation approaches to optimize absorption. Also, kinase inhibitors are frequently hard to develop using two common enabling formulation techniques- — spray-dried dispersions and lipid-based formulations.
Drug developers and patients may benefit from exploring formulations that use the lipophilic salt form of kinase inhibitors, which can improve solubility while lowering product pill burden. The lipophilic salt approach can be easily scalable and presents opportunity to use the 505(b)(2) regulatory pathway for accelerated development of approved drug products, helping reach patients sooner with a drug product that is optimized for absorption.
Need for innovative formulations
Two of the most commonly used enabling formulation techniques the pharma industry currently uses to solve the downstream problems associated with low drug solubility in water and high drug lipophilicity are amorphous spray dried dispersions (SDD) and lipid-based formulations (LBFs).4 SDDs consist of amorphous drugs within a polymer matrix, improving drug absorption by eliminating crystalline barriers to low drug solubility. They can be delivered to the patient in the form of a tablet or powder in capsule finished dosage form. Drugs that are typically well suited to the SDD approach are those that show “brick dust” type properties, showing a high melting point and low solubility in aqueous and non-aqueous solvents.
LBFs consist of drug either suspended or dissolved within a vehicle consisting of oils, surfactants and/or cosolvents and delivered via two-piece hard capsules or soft gelatin capsules. Improved drug solubility along the GI tract improves drug absorption for LBFs, while those LBFs containing dissolved drug typically show higher absorption and reduced variability in vivo as they also eliminate crystalline barriers to low drug solubility. LBFs tend to be appropriate for drugs that show “grease ball” properties, showing low solubility in water but high solubility in non-aqueous solvents such as oils.
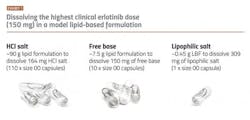
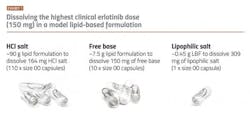
Kinase inhibitors are often not ideal for either SDD or LBF, as they frequently show intermediate brick dust/greaseball properties. This can lead to high pill burden, lower patient adherence and undesirable treatment outcomes. For example, erlotinib, currently marketed as Tarceva and FDA approved in 2004 for the treatment of metastatic non-small cell lung cancer and advanced-stage pancreatic cancer. Erlotinib would initially be considered a good candidate drug for an enabling technology approach owing to its low aqueous solubility in the small intestine (its primary site of absorption), low and variable absorption in the fasted state and clinically significant enhanced absorption when taken with food.5 However, erlotinib shows very low solubility in lipid vehicles. The LBF volume to deliver the target clinical dose translates to more than 100 regular sized capsules when using the marketed salt form (hydrochloride) (see above). When using the free base form of the drug, the situation is improved but 10 capsules are still needed for a single dose. This number of capsules is impractical for the patient, even in the treatment of cancer.
Lipophilic salt forms -— new tricks
Transforming a kinase inhibitor into lipophilic salt form is one promising approach for improving the solubility of kinase inhibitors in LBF vehicles so these drugs can access the benefits of LBF without a high pill burden.6,7,8 The conversion of drugs into salts in drug development is not new, as salts have been used for decades to improve the manufacturability of drugs and increase drug solubility in water.⁴ However, lipophilic salt forms are different in that they are specifically designed to show improved solubility in lipid-based vehicles. The net effect is that lower volumes of LBF are required to deliver the target dose. Revisiting the erlotinib example above, using the lipophilic salt form of this drug has made it possible to deliver the maximum dose in one capsule -— rather than 100.
While the lipophilic salt form takes care of the pill burden, the LBF vehicle itself takes care of the kinase inhibitor’s low solubility in water. This has been proven both in laboratory tests as well as in in vivo tests where it has been shown the LBF and lipophilic salt form work “synergistically” to increase the amount of drug dissolved in simulated intestinal conditions, which in turn translates to increased drug absorption.8 The combined use of lipophilic salts of kinase inhibitors and LBF is therefore a new approach available to chemists and formulators working in this area.
Notably, the lipophilic salt will not change the way in which the kinase inhibitor works after it is absorbed. This is because lipophilic salt formation does not involve a change in the covalent drug structure — the kinase inhibitor and the lipophilic counterion will dissociate before it crosses the intestinal membrane or before it reaches the bloodstream. The benefit of this is that lipophilic salt forms are not new chemical entities (NCEs) in the regulatory sense, meaning that existing safety and pharmacology data relating to the kinase inhibitor can be leveraged during submission. As the synthesis of lipophilic salts is, once optimized, generally high yielding and operationally simple, the approach is readily scalable for clinical development through to commercial supply.
Looking ahead
The lipophilic salt approach brings a new opportunity to chemists and formulators to deliver new kinase inhibitors currently under development in an optimized finished formulation, with reduced risk of high pill burden and absorption-related issues such as variability and food-effects. As the approach piggy-backs existing synthetic infrastructure and is combined with the well-known formulation platforms, it is readily scalable, meaning it can be pursued in new kinase inhibitor development projects that are likely to be or already are on fast-track status. Another potential benefit is that new drug applications relating to new salt forms and new formulations of FDA approved kinase inhibitors could all be filed via the 505(b)(2) pathway, which has the benefit of shorter development times and up to five years of exclusivity.
Kinase inhibitor drugs offer promise for advancing novel cancer treatments, but their low solubility can lead to challenges in terms of pill burden and adverse outcomes for patients. Common techniques for bioavailability enhancement, such as spray-dried dispersions and lipid-based formulations, are not ideal for many kinase inhibitors due to their physicochemical properties. Transforming them into their lipophilic salt form can allow the use of lipid-based formulations to increase bioavailability and lower pill burden.
References
1. Mochalkin, I. In A Kinase Platform for the Discovery of Reversible and Covalent Kinase Inhibitors, 9th Annual Drug Discovery Chemistry Conference, San Diego, CA, April 2018.
2. Herbrink, M.; Nuijen, B.; Schellens, J. H.; Beijnen, J. H. Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat. Rev. 2015, 41 (5), 412−422.
3. Herbrink, M.; Nuijen, B.; Schellens, J. H.; Beijnen, J. H. High-Tech Drugs in Creaky Formulations. Pharm. Res. 2017, 34 (9), 1751−1753.
4. Williams, H. D. et al. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65 (1), 315−499.
5. Ling, J., Fettner, S., Lum, B. L., Riek, M., & Rakhit. Effect of food on the pharmacokinetics of erlotinib in healthy individuals. Anti-cancer drugs, 19(2), 209-216.
6. Sahbaz, Y., et al. Transformation of Poorly Water-Soluble Drugs into Lipophilic Ionic Liquids Enhances Oral Drug Exposure. Mol. Pharmaceutics 2015, 12 (6), 1980−1991.
7. Williams, H. D., et al. Transformation of BCS Class I and III Drugs into Ionic Liquids and Lipophilic Salts For Enhanced Developability . J. Pharm. Sci. 2018, 107 (1), 203−216.
8. Williams, H. D., et al. “Enhancing the Oral Absorption of Kinase Inhibitors Using Lipophilic Salts and Lipid-Based Formulations.” Molecular Pharmaceutics 15, no. 12 (2018): 5678-5696.